Nat Prod Sci.
2018 Sep;24(3):181-188. 10.20307/nps.2018.24.3.181.
Analysis of Essential oil, Quantification of Six Glycosides, and Nitric Oxide Synthase Inhibition Activity in Caryopteris incana
- Affiliations
-
- 1Department of Agro-industrial Technology, Lambung Mangkurat University, Banjarbaru 70714, Indonesia.
- 2College of Pharmacy, Seoul National University, Seoul 08826, Korea.
- 3Department of Food and Nutrition, Pukyung National University, Busan 48513, Korea.
- 4Department of Oriental Medicine, Sangji University, Wonju 26339, Korea.
- 5Department of Forest Science, Sangji University, Wonju 26339, Korea.
- 6Department of Pharmaceutical Engineering, Sangji University, Wonju 26339, Korea. hjpark@sangji.ac.kr
- KMID: 2422010
- DOI: http://doi.org/10.20307/nps.2018.24.3.181
Abstract
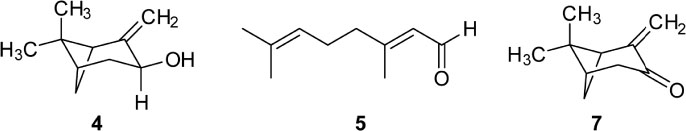
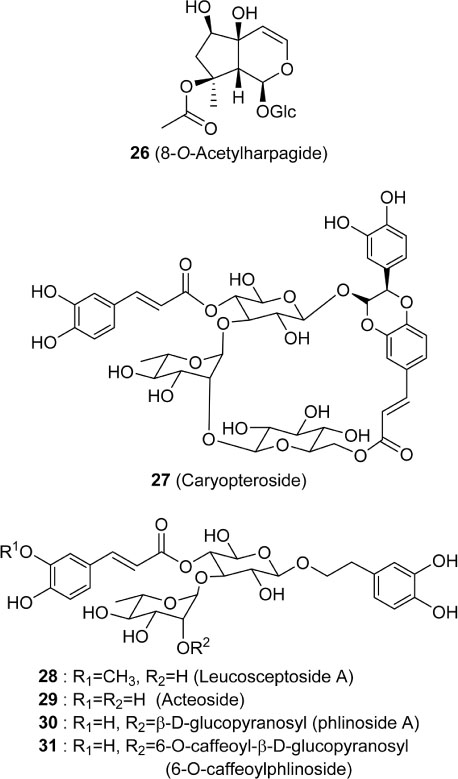
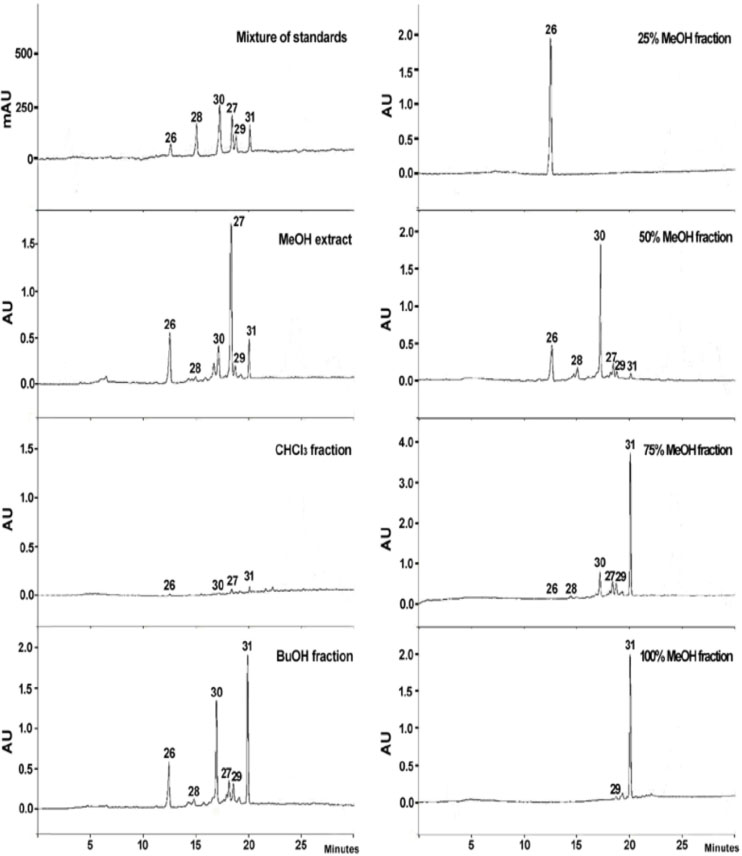
- Caryopteris incana (Verbenaceae) has been used to treat cough, arthritis, and eczema in Oriental medicine. The two fractions (CHCl₃- and BuOH fractions) and the essential oil of the plant material were subjected to the inducible nitric oxide synthase (iNOS) assay. The ICâ‚…â‚€ of the CHCl₃ fraction and the essential oil on LPS-induced macrophage RAW 264.7 cells were 16.4 µg/mL and 23.08 µg/mL, respectively. On gas chromatography (GC)-mass spectroscopy (MS) analysis, twenty-five components representing 85.5% amount of total essential oil were identified. On the chromatogram, three main substances, trans-pinocarveol, cis-citral, and pinocarvone, occupied 18.8%, 13.5% and 18.37% of total peak area. Furthermore, by HPLC-UV analysis, six compounds including one iridoid (8-O-acetylharpagide)- and five phenylethanoid glycosides (caryopteroside, acteoside, phlinoside A, 6-O-caffeoylphlinoside, and leucosceptoside A) isolated from the BuOH fraction were quantified. The content of six compounds were shown as the following order: caryopteroside (162.35 mg/g) > 8-O-acetylharpagide (93.28 mg/g) > 6-O-caffeoylphlinoside (28.15mg/g) > phlinoside (22.60mg/g) > leucosceptoside A (16.87 mg) > acteoside (7.05 mg/g).
MeSH Terms
-
Arthritis
Chromatography, Gas
Chromatography, High Pressure Liquid
Cough
Eczema
Glycosides*
Macrophages
Medicine, East Asian Traditional
Nitric Oxide Synthase Type II
Nitric Oxide Synthase*
Nitric Oxide*
Plants
RAW 264.7 Cells
Spectrum Analysis
Verbenaceae
Glycosides
Nitric Oxide
Nitric Oxide Synthase
Nitric Oxide Synthase Type II
Figure
Reference
-
1. Moncada S, Palmer RMJ, Higgs EA. Pharmacol Rev. 1991; 43:109–142.2. Baydoun AR, Foale RD, Mann GE. Br J Pharmacol. 1993; 109:987–991.3. Pacher P, Beckman JS, Liaudet L. Physiol Rev. 2007; 87:315–424.4. Park S, Son MJ, Yook CS, Jin CB, Lee YS, Kim HJ. Phytochemistry. 2014; 101:83–89.5. Zhao DP, Matsunami K, Otsuka H. J Nat Med. 2009; 63:241–247.6. Yoshikawa K, Harada A, Iseki K, Hashimoto T. J Nat Med. 2014; 68:231–235.7. Gao JJ, Igalshi K, Nukina M. Biosci Biotechnol Biochem. 1999; 63:983–988.8. Choi J, Shin KM, Park HJ, Jung HJ, Kim HJ, Lee YS, Rew JH, Lee KT. Planta Med. 2004; 70:1027–1032.9. Cui Q, Pan Y, Xu X, Zhang W, Wu X, Qu S, Liu X. Fitoterapia. 2016; 109:67–74.10. Froelich S, Gupta MP, Siems K, Jenett-Siems K. Braz J Pharmacogn. 2008; 18:517–520.11. Schlauer J, Budzianowski J, Kukulczanka K, Ratajczak L. Acta Soc Bot Pol. 2004; 73:9–15.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nitric Oxide Synthase Inhibition Alters Extracellular Glutamate Concentration after Global Cerebral Ischemia
- Role of nitric oxide and distribution of nitric oxide synthase in the gustatory system
- Isolation of Constituents with Nitric Oxide Synthase Inhibition Activity from Phryma leptostachya var. asiatica
- Distribution of Nitric Oxide Synthase Isoforms in Perioral Exocrine Glands in Rats
- Involvement of Fibronectin in the Migration of Macrophage and Expression of Nitric Oxide Synthase in the BCG induced Inflammatory Sites in Rat Bladder