Nat Prod Sci.
2018 Sep;24(3):155-158. 10.20307/nps.2018.24.3.155.
Meliglabrin, A New Flavonol Derivative from the leaves of Melicope glabra (Blume) T.G. Hartley
- Affiliations
-
- 1Natural Products Chemistry Research Group, Organic Chemistry Division, Department of Chemistry, Faculty of Science and Technology, Universitas Airlangga, Surabaya, Indonesia. mulyadi-t@fst.unair.ac.id
- KMID: 2422006
- DOI: http://doi.org/10.20307/nps.2018.24.3.155
Abstract
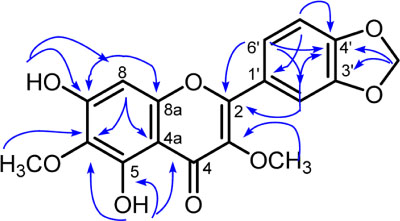
- A new flavonol derivative, meliglabrin (1) along with three known flavonols, ternatin (2), meliternatin (3), and 5,4"²-dihydroxy-3,7,3"²-trimethoxyflavon (4) were isolated from the leaves of Melicope glabra (Blume) T.G. Hartley. Their structures were determined using extensive spectroscopic methods, including UV, IR, HRESIMS, 1D and 2D NMR. Compounds 1 - 4 were evaluated for their cytotoxicity against murine leukemia P-388 cells, compound 4 showed moderate activity.
Figure
Reference
-
1. Hartley T. Sandakanian. 1994; 4:47–74.2. Nakashima K, Oyama M, Ito T, Akao Y, Witono JR, Darnaedi D, Tanaka T, Murata J, Iinuma M. Tetrahedron. 2012; 68:2421–2428.3. Tanjung M, Saputri RD, Wahjoedi RA, Tjahjandarie TS. Molbank. 2017; M939:1–5.4. Simonsen HT, Adsersen A, Bremner P, Heinrich M, Wagner Smitt U, Jaroszewski JW. Phytother Res. 2004; 18:542–545.5. Chung LY, Yap KF, Goh SH, Mustafa MR, Imiyabir Z. Phytochemistry. 2008; 69:1548–1554.6. Kassim NK, Rahmani M, Ismail A, Sukari MA, Ee GC, Nasir NM, Awang K. Food Chem. 2013; 139:87–92.7. Oyama M, Nakashima K, Kamiya T, Haba M, Ito T, Murata H, Tanaka T, Adachi T, Iinuma M, Kinoshita T. Phytochem Lett. 2013; 6:215–218.8. Cambie RC, Pan YJ, Bowden BF. Biochem Syst Ecol. 1996; 24:461–462.9. Chung LY, Yap KF, Goh SH, Mustafa MR, Imiyabir Z. Phytochemistry. 2008; 69:1548–1554.10. Higa M, Imamura M, Ogihara K, Suzuka T. Chem Pharm Bull. 2013; 61:384–389.11. Tanjung M, Hakim EH, Syah YM. Chem Nat Compd. 2017; 53:215–218.12. Tanjung M, Hakim EH, Elfahmi Latip J, Syah YM. Nat Prod Commun. 2012; 7:1309–1310.13. Tjahjandarie TS, Pudjiastuti P, Saputri RD, Tanjung M. J Chem Pharm Res. 2014; 6:786–790.14. Hou RS, Duh CY, Wang SK, Chang TT. Phytochemistry. 1994; 35:271–272.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Melixyloidin, A New Acridone Alkaloid from Melicope xanthoxyloides Leaves
- Isolation of Quercetin and Isorhamnetin Derivatives and Evaluation of Anti-microbial and Anti-inflammatory Activities of Persicaria glabra
- Xanthone and Flavonoid Derivatives from the Leaves of Maclura tricuspidata with Antioxidant and Anti-tyrosinase Activity
- Anti-Helicobacter pylori activity of acomplex mixture of Lactobacillus paracasei HP7 including the extract of Perilla frutescens var. acuta and Glycyrrhiza glabra
- A New Cytotoxic Compound from Methanol Extract of Koordersiodendron pinnatum Merr. Leaves