Nutr Res Pract.
2010 Feb;4(1):11-15.
Effect of ethanol extracts from red pepper seeds on antioxidative defense system and oxidative stress in rats fed high-fat, high-cholesterol diet
- Affiliations
-
- 1School of Food science, International University of Korea, Sangmun-ri, Munsan-eup, Jinju-si, Gyeongnam 660-759, Korea. jhappychoi@hanmail.net
- 2Korea Food Research Institute, Gyeonggi 463-746, Korea.
Abstract
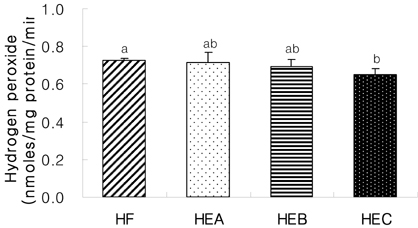
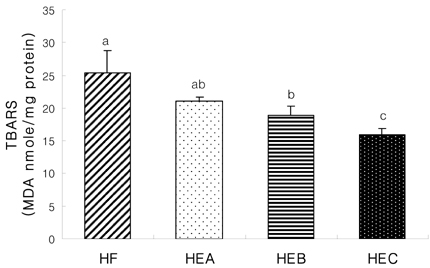
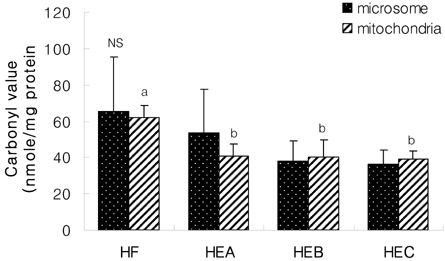
- The purpose of the present study was to investigate the effect of ethanol extracts from red pepper seeds on the antioxidative defense system and oxidative stress in rats fed a high fat . high cholesterol diet. Rats were divided into four experimental groups which were composed of high fat . high cholesterol diet group (HF), high fat . high cholesterol diet with 0.1% ethanol extracts from red pepper seeds supplemented group (HEA), high fat . high cholesterol diet with 0.2% ethanol extracts from red pepper seeds supplemented group (HEB) and high fat.high cholesterol diet with 0.5% ethanol extracts from red pepper seeds supplemented group (HEC). Supplementation of ethanol extracts from red pepper seeds groups (HEA, HEB and HEC) resulted in significantly increased activities of hepatic glutathione peroxidase and catalase. Hepatic superoxide radical contents in microsome and mitochondria were significantly reduced in the groups supplemented with red pepper seeds ethanol extracts. Hepatic hydrogen peroxide content in the mitochondria was reduced in ethanol extracts from red pepper seeds supplemented groups. TBARS values in the liver were reduced in red pepper seeds ethanol extracts supplemented groups. Especially, HEB and HEC groups were significantly decreased compared to the HF group. Hepatic carbonyl values were significantly reduced in mitochondria in these supplemented groups. These results suggest that red pepper seeds ethanol extracts may reduce oxidative damage, by activation of antioxidative defense system in rats fed high fat . high cholesterol diets.
Keyword
MeSH Terms
-
Animals
Capsicum
Catalase
Cholesterol
Diet
Ethanol
Glutathione Peroxidase
Hydrogen Peroxide
Liver
Microsomes
Mitochondria
Oxidative Stress
Rats
Seeds
Superoxides
Thiobarbituric Acid Reactive Substances
Catalase
Cholesterol
Ethanol
Glutathione Peroxidase
Hydrogen Peroxide
Superoxides
Thiobarbituric Acid Reactive Substances
Figure
Reference
-
1. Halliwell B, Gutteridge JMC. Free radicals, ageing, and disease. Free radicals in biology and medicine. 1996. London. England: Clarendon Press Publishing Co;416.2. Hammers HD, Martin S, Fedesrlin K, Geisen K, Brownlee M. Aminoguanidine treatment inhibit the development of experimental diabetic retinopathy. Proc Natl Acad Sci U S A. 1991. 8:11555–11558.3. Park GY, Rhee SJ, Im JG. Effects of green tea catechin on cytochrome P450, xanthine oxidase activities in liver and liver damage in streptozocin induced diabetic rats. J Korean Soc Food Sci Nutr. 1997. 26:901–907.4. Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta. 1998. 1366:53–67.
Article5. Block G, Langseth L. Antioxidant vitamins and disease prevention. Food Technol. 1994. 48:80–85.6. Manjeshwar SB, Ganesh CJ, Shaial KR, Kiran BS. Evaluation of nitric oxide scavenging activity of certain spices in vitro: A preliminary study. Nahrung. 2003. 47:261–264.
Article7. Hasler CM, Kundrat S, Wool D. Functional foods and cardiovascular disease. Curr Atheroscler Rep. 2000. 2:467–475.
Article8. Lee KJ, Han JS, Lee SW, Park CR. Investigation of Lipids in Hot Pepper I. Neutral lipids of hot pepper seeds. Korean Journal of Food Science and Technology. 1975. 7:91–95.9. Sim KH, Han YS. The antimutagenic and antioxidant effects of red pepper and red pepper pericarp (Capsicum annum L.). Journal of Food Science and Nutrition. 2007. 12:273–278.
Article10. Morimoto C, Tsujita T, Okuda H. Ephinephrine-induced lipolysis in rats fat cell from visceral and subcutaneous sites: role of hormone-sensitive and lipid droplets. J Lipid Res. 1997. 38:132–138.
Article11. Slater TF, Sawyer BC. The Stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions on rat liver fractions in vivo. Biochem J. 1971. 123:805–814.
Article12. Lowry OH, Roseborough NJ, Ferr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951. 193:265–275.
Article13. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974. 47:469–474.
Article14. Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium deficient rat liver. Biochem Biophys Res Commun. 1976. 71:952–958.15. Aebi H, Wyss SR, Scherz B, SKvaril F. Heterogeneity of erythrocyte catalase. Isolation and characterixation of normal and variant erythrocyte catalase and their subunits. Eur J Biochem. 1974. 48:137–145.
Article16. Azzi A, Montecucco C, Richter C. The use of acetylated ferric cytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophy Res Commun. 1975. 65:597–603.
Article17. Gay C, Gebicki JM. A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal Biochem. 2000. 284:217–220.
Article18. Levin RL, Garland D, Oliver CN, Amici A, Climent I, lenz AG, Ahn B, Shatiel S, Stadtman ER. Determination of carbonyl content intoxidatively modified proteins. Meth Enzymol. 1990. 186:464–478.19. Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978. 90:37–43.
Article20. Sreel RGD, Torrie JH. Principles and procedures of statistics. 1990. New York: McGrow Hill.21. Ki HS, Han YS. Antioxidant activities of red pepper (Capsicum annuum) pericarp and seed extracts. International Journal of Food Science & Technology. 2008. 43:1813–1823.
Article22. Lee YJ, Han JP. Antioxidant activities and nitrite scavenging abilities of extracts from Ulmus devidianan. J Korean Soc Food Sci Nutr. 2000. 29:893–899.23. Fang YZ, Yang S, Wu G. Free radicals, antioxidants and nutrition. Nutrition. 2002. 18:872–879.
Article24. Lee SJ, Choi SK, Seo JS. Grape skin improves antioxidant capacity in rats fed high fat diet. Nutr Res Pract. 2009. 3:279–285.
Article25. Rocha KK, Souza GA, Ebaid GX, Seivia FRF, Cataneo AC, Novelli ELB. Resveratrol toxicity: Effects on risk factors for atherosclerosis and hepatic oxidative stress in standard an high-fat diets. Food Chem Toxicol. 2009. 47:1362–1367.
Article26. Park SY, Bok SH, Jeon SM, Park YB, Lee SJ, Jeong TS, Choi MS. Effect of rutin and tannic acid supplements on cholesterol metabolism in rats. Nutr Res. 2002. 22:283–295.
Article27. Ku KH, Choi EJ, Park WS. Functional Activity of Water and Ethanol Extracts from Red Pepper (Capsicum annuum L.) Seeds. Journal of the Korean Society of Food Science and Nutrition. 2008. 37:1357–1362.
Article28. Lee JH, Lee SR. Analysis of phenolic substances content in Korean plant foods. Journal of the Korean Society of Food Science and Nutrition. 1994. 26:310–316.29. Cuvelier ME, Richard H, Berset C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J Am Oil Chem Soc. 1996. 73:645–652.
Article30. Jimenez-Escrig A, Rincon M, Pulido , Saura-Clixto F. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J Agric Food Chem. 2001. 49:5489–5493.
Article31. Fridovich I. Superoxide dismutase. An adaptation to a paramagnetic gas. J Biol Chem. 1989. 264:7761–7764.32. Sim KH, Han YS. Effect of red pepper seed on Kimchi antioxidant activity during fermentation. Food Sci Biotechnol. 2008. 17:295–301.33. Sohal RS, Allen RG. Oxidative stress as a causal factor indifferentiation and aging: A unifying hypothesis. Exp Gerontol. 1990. 25:499–522.
Article34. Feillet-Coudray C, Sutra T, Fouret G, Ramos J, Wrutniak-Cabello C, Cabello G, Cristol JP, Coudray C. Oxidative stress in rats fed a high-fat high-sucrose diet and preventive effect of polyphenols: Involvement of mitochondrial and NAD(P)H oxidase systems. Free Radic Biol Med. 2009. 46:624–632.
Article35. Fan J, Zeng M, Li J. Correlation between hepatic fat, lipid peroxidation and hepatic fibrosis in rats chronically fed with ethanol and/or high fat diet. Zhonghua Nei Ke Za Zhi. 1997. 36:808–811.36. Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005. 191:318–325.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Water Extracts of Red Pepper Seeds Powder on Antioxidative Enzyme Activities and Oxidative Damage in Rats Fed High-Fat and High-Cholesterol Diets
- Effect of Seeds Extract of Paeonia Lactiflora on Antioxidative System and Lipid Peroxidation of Liver in Rats Fed High-Cholesterol Diet
- Antioxidative and antiproliferative activities of ethanol extracts from pigmented giant embryo rice (Oryza sativa L. cv. Keunnunjami) before and after germination
- Effect of Forsythia Viridissima Extracts on Antioxidative System and Lipid Peroxidation of Liver in Rats Fed High-Cholesterol Diet
- Effects of vitamin C and E supplementation on oxidative stress and liver toxicity in rats fed a low-fat ethanol diet