J Breast Cancer.
2018 Sep;21(3):315-320. 10.4048/jbc.2018.21.e35.
Evaluating the Prediction of Breast Cancer Survival Using Lymph Node Ratio
- Affiliations
-
- 1Department of Orthopaedics, University of Utah School of Medicine, Salt Lake City, USA. Man.Hung@hsc.utah.edu
- 2Study Design and Biostatistics Center, University of Utah School of Medicine, Salt Lake City, USA.
- 3Huntsman Cancer Institute, University of Utah, Salt Lake City, USA.
- KMID: 2421372
- DOI: http://doi.org/10.4048/jbc.2018.21.e35
Abstract
- PURPOSE
Previous oncological studies showed that lymph node ratio (LNR) (ratio of number of lymph nodes that tested positive for metastasis to the total number of lymph nodes examined) is a negative indicator of cancer survival. The American Joint Committee on Cancer (AJCC) staging system incorporates tumor size, lymph node involvement, and metastasis in a comprehensive model of cancer progression, but LNR alone has been shown to outperform the AJCC system in prognostic and survival predictions for various types of cancer. The effectiveness of LNR has not been evaluated in breast cancer staging. Evaluating LNR for predicting cancer staging in breast cancer has the potential to improve treatment recommendations.
METHODS
The Surveillance, Epidemiology, and End Results dataset was used to identify 10,655 breast cancer patients who underwent nodal evaluation from 2010 to 2013, and their LNRs were calculated. Descriptive statistics of lymph node evaluation in the patients are provided. Logistic regression with LNR as the continuous independent variable was conducted to determine whether LNR could predict cancer progression, coded as regional or distant. Analysis was conducted using SPSS version 24.
RESULTS
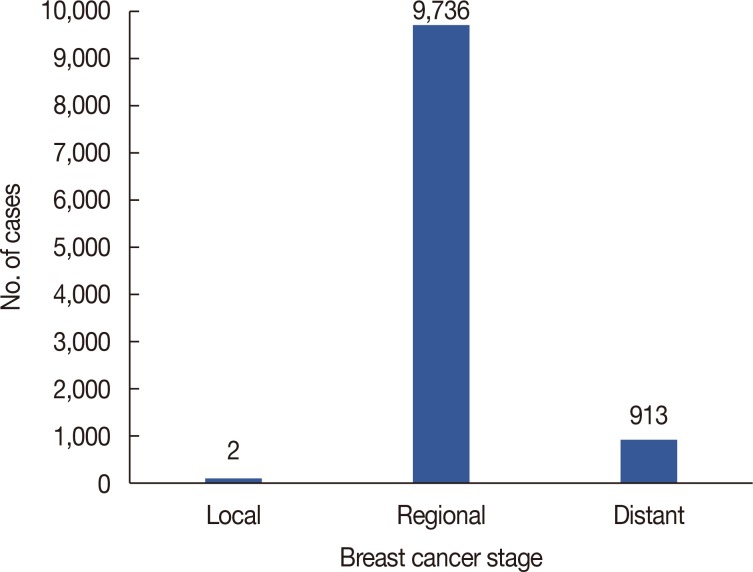
Patient's mean age was 59.43±18.62. Logistic regression analysis revealed that for every 1.3% increase in LNR, the odds of falling into the distant stage of the TNM staging system increased by 13.7% (odds ratio, 14.73; 95% confidence interval, 12.00-18.08).
CONCLUSION
LNR, while correlated with breast cancer staging, serves as a better predictor of survival. Precision staging can influence treatment modality, and improved treatments can significantly improve quality of life. Additional research and diagnostic examinations using LNR as a potential tool for accurate staging in breast cancer patients are warranted.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30. PMID: 26742998.
Article2. Brandt J, Garne JP, Tengrup I, Manjer J. Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol. 2015; 13:33. PMID: 25889186.
Article3. Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015; 314:1599–1614. PMID: 26501536.4. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.
Article5. Greene FL. American Joint Committee on Cancer. American Cancer Society. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag;2002.6. Chavez-MacGregor M, Mittendorf EA, Clarke CA, Lichtensztajn DY, Hunt KK, Giordano SH. Incorporating tumor characteristics to the American Joint Committee on Cancer breast cancer staging system. Oncologist. 2017; 22:1292–1300. PMID: 28592619.
Article7. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:290–303. PMID: 28294295.
Article8. Amin MB. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th ed. New York: Springer;2017. p. 589–636.9. Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015; 107:djv048. PMID: 25825511.
Article10. Gándara-Cortes M, Vázquez-Boquete Á, Fernández-Rodríguez B, Viaño P, Ínsua D, Seoane-Seoane A, et al. Breast cancer subtype discrimination using standardized 4-IHC and digital image analysis. Virchows Arch. 2018; 472:195–203. PMID: 28825136.
Article11. Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018; 27:619–626. PMID: 29593010.
Article12. Parise CA, Caggiano V. Risk of mortality of node-negative, ER/PR/HER2 breast cancer subtypes in T1, T2, and T3 tumors. Breast Cancer Res Treat. 2017; 165:743–750. PMID: 28689363.
Article13. Yang ZJ, Yu Y, Chi JR, Guan M, Zhao Y, Cao XC. The combined pN stage and breast cancer subtypes in breast cancer: a better discriminator of outcome can be used to refine the 8th AJCC staging manual. Breast Cancer. 2018; 25:315–324. PMID: 29353447.
Article14. Lee YC, Yang PJ, Zhong Y, Clancy TE, Lin MT, Wang J. Lymph node ratio-based staging system outperforms the seventh AJCC system for gastric cancer: validation analysis with national Taiwan University Hospital Cancer Registry. Am J Clin Oncol. 2017; 40:35–41. PMID: 25089533.15. Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007; 141:610–618. PMID: 17462460.
Article16. Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003; 21:2912–2919. PMID: 12885809.
Article17. Kutlu OC, Watchell M, Dissanaike S. Metastatic lymph node ratio successfully predicts prognosis in Western gastric cancer patients. Surg Oncol. 2015; 24:84–88. PMID: 25912951.
Article18. Wang J, Hassett JM, Dayton MT, Kulaylat MN. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol. 2008; 15:1600–1608. PMID: 18327530.
Article19. Conci S, Ruzzenente A, Sandri M, Bertuzzo F, Campagnaro T, Bagante F, et al. What is the most accurate lymph node staging method for perihilar cholangiocarcinoma? Comparison of UICC/AJCC pN stage, number of metastatic lymph nodes, lymph node ratio, and log odds of metastatic lymph nodes. Eur J Surg Oncol. 2017; 43:743–750. PMID: 28094085.
Article20. Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006; 24:2910–2916. PMID: 16782931.
Article21. Wu SG, Wang Y, Zhou J, Sun JY, Li FY, Lin HX, et al. Number of negative lymph nodes should be considered for incorporation into staging for breast cancer. Am J Cancer Res. 2015; 5:844–853. PMID: 25973321.22. Vinh-Hung V, Nguyen NP, Cserni G, Truong P, Woodward W, Verkooijen HM, et al. Prognostic value of nodal ratios in node-positive breast cancer: a compiled update. Future Oncol. 2009; 5:1585–1603. PMID: 20001797.
Article23. Safavi A, Kaviani A, Mohammadzadeh N, Zand S, Elahi A, Krag DN. Breast cancer prognostication by pathologic node staging (pN-staging) system versus lymph node ratio (LNR): a critical review of conflicts with number of nodes, z-0011 trial, staging cut-points, neo-adjuvant therapy, and survival estimation. Arch Breast Cancer. 2017; 4:110–123.24. Tsai J, Bertoni D, Hernandez-Boussard T, Telli ML, Wapnir IL. Lymph node ratio analysis after neoadjuvant chemotherapy is prognostic in hormone receptor-positive and triple-negative breast cancer. Ann Surg Oncol. 2016; 23:3310–3316. PMID: 27401442.
Article25. Tausch C, Taucher S, Dubsky P, Seifert M, Reitsamer R, Kwasny W, et al. Prognostic value of number of removed lymph nodes, number of involved lymph nodes, and lymph node ratio in 7502 breast cancer patients enrolled onto trials of the Austrian Breast and Colorectal Cancer Study Group (ABCSG). Ann Surg Oncol. 2012; 19:1808–1817. PMID: 22207051.
Article26. Kittaneh M, Montero AJ, Glück S. Molecular profiling for breast cancer: a comprehensive review. Biomark Cancer. 2013; 5:61–70. PMID: 24250234.
Article27. Kwa M, Makris A, Esteva FJ. Clinical utility of gene-expression signatures in early stage breast cancer. Nat Rev Clin Oncol. 2017; 14:595–610. PMID: 28561071.
Article28. Solak M, Turkoz FP, Keskin O, Aksoy S, Babacan T, Sarici F, et al. The lymph node ratio as an independent prognostic factor for non-metastatic node-positive breast cancer recurrence and mortality. J BUON. 2015; 20:737–745. PMID: 26214625.29. Liu D, Chen Y, Deng M, Xie G, Wang J, Zhang L, et al. Lymph node ratio and breast cancer prognosis: a meta-analysis. Breast Cancer. 2014; 21:1–9. PMID: 24101545.
Article30. National Cancer Institute. Scope of regional lymph node surgery-SEER documentation. Accessed February 6th, 2018. https://seer.cancer.gov/seerstat/variables/seer/regional_ln/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Number of Removed Lymph Nodes for an Acceptable False Negative Rate in Sentinel Lymph Node Biopsy for Breast Cancer
- Ultrasonography for Staging Axillary Lymph Node in Breast Cancer Patients
- Quadrantectomy and axillary lymph node dissection on breast cancer
- The Impact of the Ratio of Positive Nodes to Removed Nodes on Recurrence and Overall Survival in Node Positive Breast Cancer Patients
- Optimized Criteria for Sentinel Lymph Node Biopsy in Patients with Clinically Node Negative Breast Cancer