Anat Cell Biol.
2018 Jun;51(2):105-112. 10.5115/acb.2018.51.2.105.
CD57 (Leu-7, HNK-1) immunoreactivity seen in thin arteries in the human fetal lung
- Affiliations
-
- 1Department of Anatomy, Tokyo Dental College, Tokyo, Japan. yamamotomasahito@tdc.ac.jp
- 2Department of Anatomy, Wuxi Medical School, Jiangnan University, Wuxi, China.
- 3Division of Internal Medicine, Iwamizawa Asuka Hospital, Iwamizawa, Japan.
- 4Department of Dentistry, Jikei University School of Medicine, Tokyo, Japan.
- KMID: 2421174
- DOI: http://doi.org/10.5115/acb.2018.51.2.105
Abstract
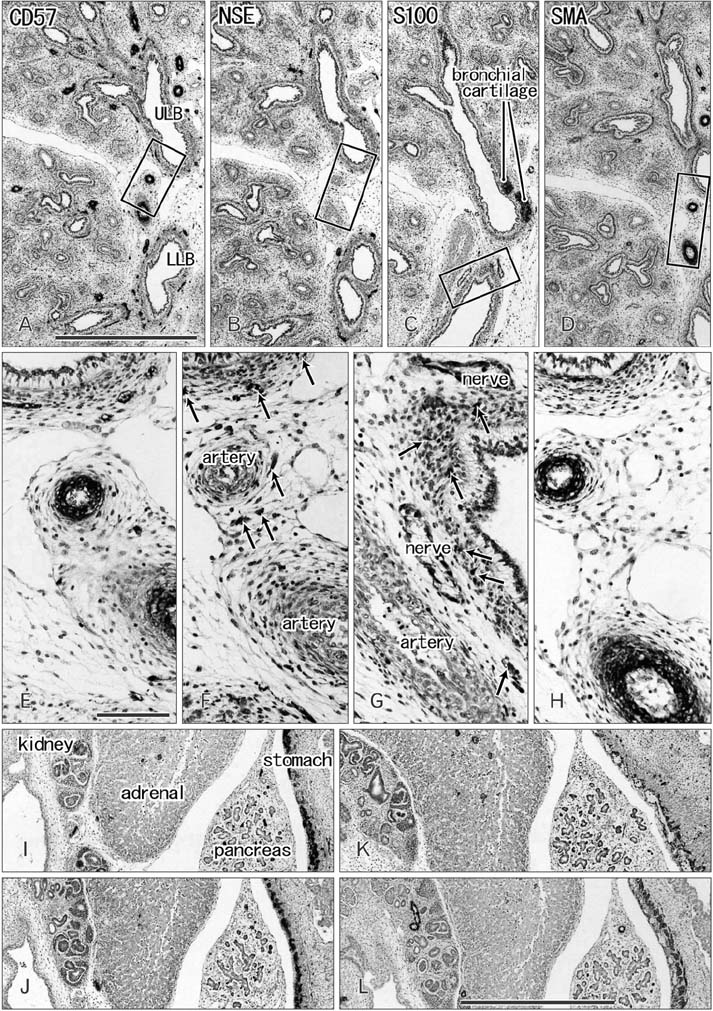
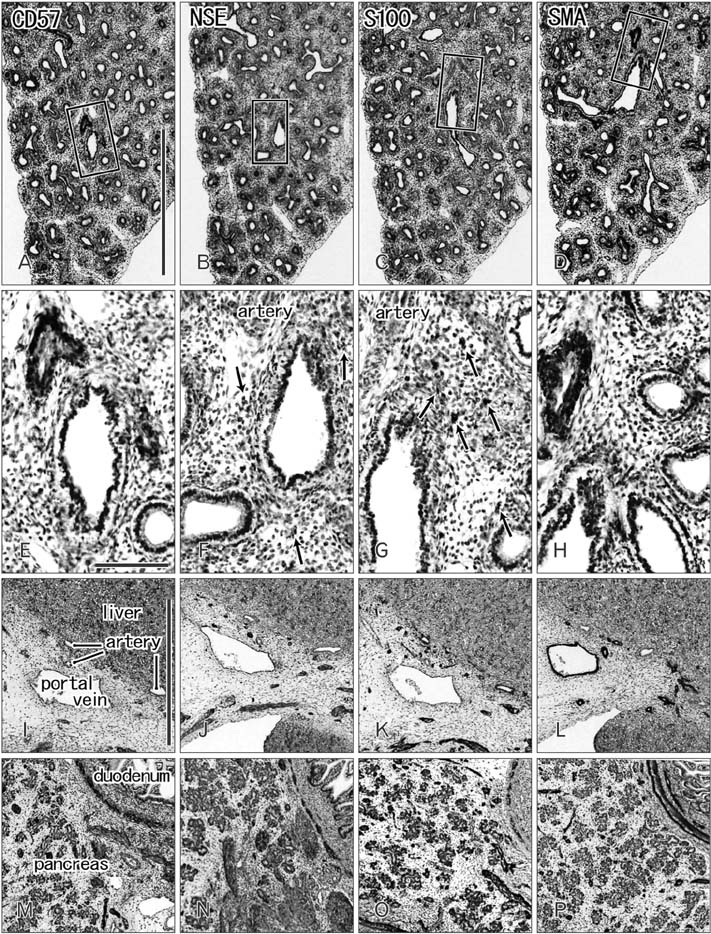
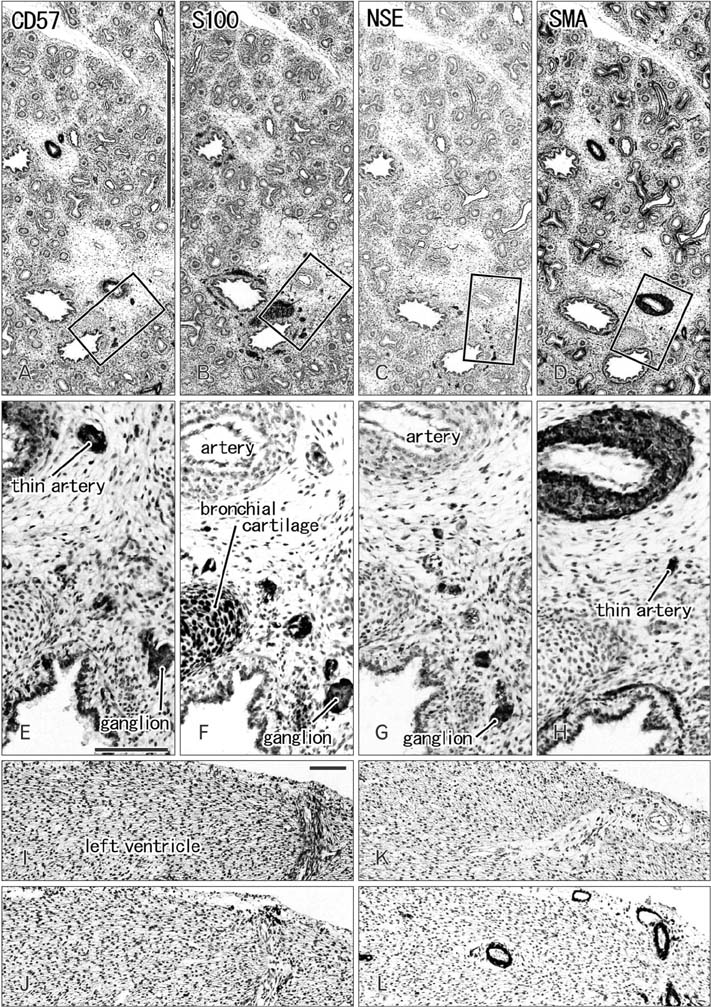
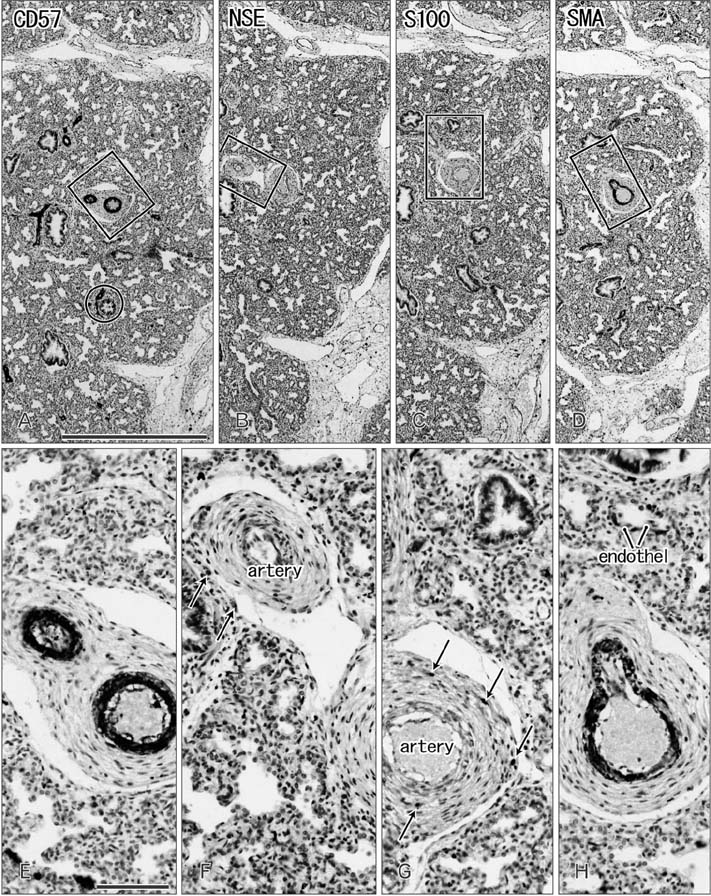
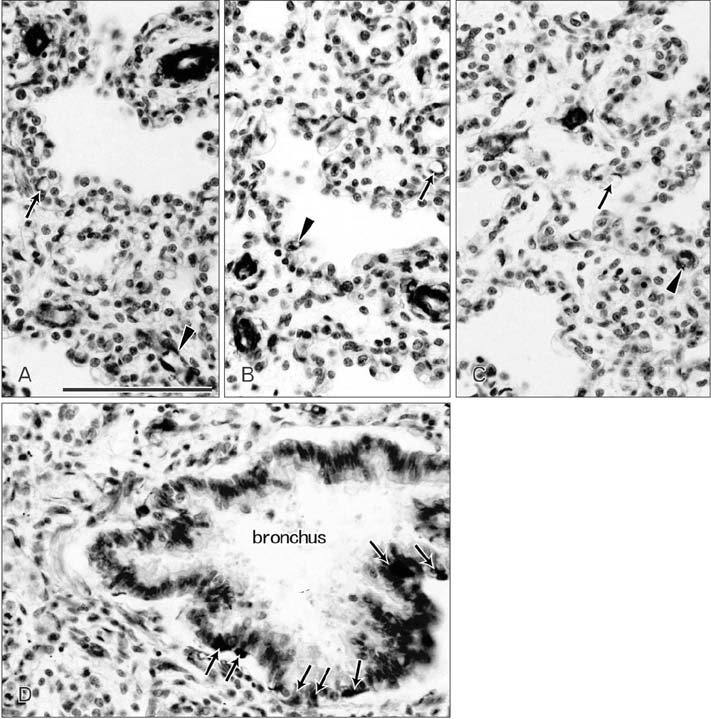
- CD57 (synonyms: Leu-7, HNK-1) is a well-known marker of nerve elements including the conductive system of the heart, as well as natural killer cells. In lung specimens from 12 human fetuses at 10-34 weeks of gestation, we have found incidentally that segmental, subsegmental, and more peripheral arteries strongly expressed CD57. Capillaries near developing alveoli were often or sometimes positive. The CD57-positive tissue elements within intrapulmonary arteries seemed to be the endothelium, internal elastic lamina, and smooth muscle layer, which corresponded to tissue positive for a DAKO antibody reactive with smooth muscle actin we used. However, the lobar artery and pulmonary arterial trunk as well as bronchial arteries were negative. Likewise, arteries in and along any abdominal viscera, as well as the heart, thymus, and thyroid, did not express CD57. Thus, the lung-specific CD57 reactivity was not connected with either of an endodermal- or a branchial arch-origin. CD57 antigen is a sugar chain characterized by a sulfated glucuronic acid residue that is likely to exist in some glycosphingolipids. Therefore, a chemical affinity or an interaction might exist between CD57-positive arterioles and glycosphingolipids originating from alveoli, resulting in acceleration of capillary budding to make contact with the alveolar wall. CD57 might therefore be a functional marker of the developing air-blood interface that characterizes the fetal lung at the canalicular stage.
Keyword
- CD57; Leu-7; HNK-1; Lung; Human fetus
MeSH Terms
Figure
Reference
-
1. Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci. 2004; 5:195–208.
Article2. Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev Biol. 1986; 115:44–55.
Article3. Verberne ME, Gittenberger-de Groot AC, Poelmann RE. Lineage and development of the parasympathetic nervous system of the embryonic chick heart. Anat Embryol (Berl). 1998; 198:171–184.
Article4. Ikeda T, Iwasaki K, Shimokawa I, Sakai H, Ito H, Matsuo T. Leu-7 immunoreactivity in human and rat embryonic hearts, with special reference to the development of the conduction tissue. Anat Embryol (Berl). 1990; 182:553–562.
Article5. Aoyama N, Tamaki H, Kikawada R, Yamashina S. Development of the conduction system in the rat heart as determined by Leu-7 (HNK-1) immunohistochemistry and computer graphics reconstruction. Lab Invest. 1995; 72:355–366.6. Vassiliadou N, Bulmer JN. Immunohistochemical evidence for increased numbers of ‘classic’ CD57+ natural killer cells in the endometrium of women suffering spontaneous early pregnancy loss. Hum Reprod. 1996; 11:1569–1574.
Article7. O'Rahilly R, Müller F. Human embryology and teratology. 2nd ed. New York: Wiley-Liss;1996. p. 265–271.8. Hayashi S, Murakami G, Ohtsuka A, Itoh M, Nakano T, Fukuzawa Y. Connective tissue configuration in the human liver hilar region with special reference to the liver capsule and vascular sheath. J Hepatobiliary Pancreat Surg. 2008; 15:640–647.
Article9. Miyake N, Hayashi S, Kawase T, Cho BH, Murakami G, Fujimiya M, Kitano H. Fetal anatomy of the human carotid sheath and structures in and around it. Anat Rec (Hoboken). 2010; 293:438–445.
Article10. Haimoto H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-alpha and S100-beta proteins in normal nonnervous human tissues. Lab Invest. 1987; 57:489–498.11. Matsunou H, Konishi F. Papillary-cystic neoplasm of the pancreas: a clinicopathologic study concerning the tumor aging and malignancy of nine cases. Cancer. 1990; 65:283–291.
Article12. Yantiss RK, Chang HK, Farraye FA, Compton CC, Odze RD. Prevalence and prognostic significance of acinar cell differentiation in pancreatic endocrine tumors. Am J Surg Pathol. 2002; 26:893–901.
Article13. Kawamoto A, Kitamura K, Yamamoto M, Murakami G, Abe S, Katori Y. Morphological differences in innervation between mucous glands and serous glands: a quantitative histological study using the sublingual glands of elderly humans. Acta Otolaryngol. 2015; 135:942–949.
Article14. Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Parrinello CM, Hunt P, Deeks SG, Hodis HN. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011; 217:207–213.
Article15. Schumacher H, Kaiser E, Schnabel PA, Sykora J, Eckstein HH, Allenberg JR. Immunophenotypic characterisation of carotid plaque: increased amount of inflammatory cells as an independent predictor for ischaemic symptoms. Eur J Vasc Endovasc Surg. 2001; 21:494–501.
Article16. Winchester R, Giles JT, Nativ S, Downer K, Zhang HZ, Bag-Ozbek A, Zartoshti A, Bokhari S, Bathon JM. Association of elevations of specific T cell and monocyte subpopulations in rheumatoid arthritis with subclinical coronary artery atherosclerosis. Arthritis Rheumatol. 2016; 68:92–102.
Article17. Yu HT, Youn JC, Lee J, Park S, Chi HS, Lee J, Choi C, Park S, Choi D, Ha JW, Shin EC. Characterization of CD8(+)CD57(+) T cells in patients with acute myocardial infarction. Cell Mol Immunol. 2015; 12:466–473.
Article18. Duvar S, Peter-Katalinić J, Hanisch FG, Müthing J. Isolation and structural characterization of glycosphingolipids of in vitro propagated bovine aortic endothelial cells. Glycobiology. 1997; 7:1099–1109.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oral Administration of Glycine and Leucine Dipeptides Improves Skin Hydration and Elasticity in UVB-Irradiated Hairless Mice
- The Prognostic Significance of Intratumoral Natural Killer Cells in Colorectal Cancer
- Peripheral T Cell Subpopulation in Patients with Pulmonary Tuberculosis
- Association of CD57+ Natural Killer Cells with Better Overall Survival in DLBCL Patients
- Histochemical Studies of Fetal Arteries of Koreans with Special Reference to Atherogenesis in Adults