J Vet Sci.
2018 Sep;19(5):600-607. 10.4142/jvs.2018.19.5.600.
Pharmacokinetics of enrofloxacin HCl-2Hâ‚‚O (Enro-C) in dogs and pharmacokinetic/pharmacodynamic Monte Carlo simulations against Leptospira spp.
- Affiliations
-
- 1Department of Physiology and Pharmacology, National Autonomous University of Mexico (UNAM), Mexico City 04510, Mexico. liliago@unam.mx
- 2Department of Genetics and Biostatistics, National Autonomous University of Mexico (UNAM), Mexico City 04510, Mexico.
- KMID: 2420928
- DOI: http://doi.org/10.4142/jvs.2018.19.5.600
Abstract
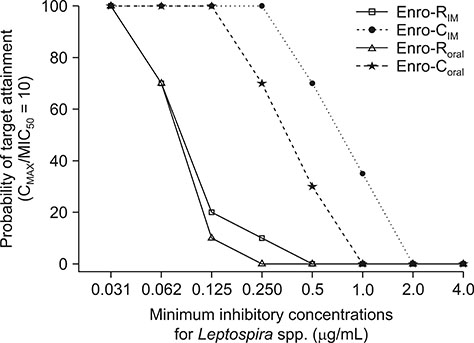
- Pharmacokinetic/pharmacodynamic (PK/PD) ratios of reference enrofloxacin (Enro-R) and enrofloxacin as HCl-2Hâ‚‚O (Enro-C), as well as Monte Carlo simulations based on composite MICâ‚…â‚€ and MIC₉₀ (MIC, minimum inhibitory concentration) vs. Leptospira spp., were carried out in dogs after their intramuscular (IM) or oral administration (10 mg/kg). Plasma determination of enrofloxacin was achieved by means of high-performance liquid chromatography. Maximum plasma concentration values after oral administration were 1.47 ± 0.19 µg/mL and 5.3 ± 0.84 µg/mL for Enro-R and Enro-C, respectively, and 1.6 ± 0.12 µg/mL and 7.6 ± 0.93 µg/mL, respectively, after IM administration. Areas under the plasma vs. time concentration curve in 24 h (AUC₀₋₂₄) were 8.02 µg/mL/h and 36.2 µg/mL/h for Enro-R(oral) and Enro-C(oral), respectively, and 8.55 ± 0.85 µg/mL/h and 56.4 ± 6.21 µg/mL/h after IM administration of Enro-R and Enro-C, respectively. The PK/PD ratios and Monte Carlo simulations obtained with Enro-C, not Enro-R, indicated that its IM administration to dogs will result in therapeutic concentrations appropriate for treating leptospirosis. This is the first time enrofloxacin has been recommended to treat this disease in dogs.
Keyword
MeSH Terms
Figure
Reference
-
1. Asín-Prieto E, Rodríguez-Gascón A, Isla A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J Infect Chemother. 2015; 21:319–329.
Article2. Bergmann M, Llewellyn JR, Hartmann K. [Diagnosis of leptospirosis in dogs]. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2017; 45:170–177. German.3. Bidgood TL, Papich MG. Plasma and interstitial fluid pharmacokinetics of enrofloxacin, its metabolite ciprofloxacin, and marbofloxacin after oral administration and a constant rate intravenous infusion in dogs. J Vet Pharmacol Ther. 2005; 28:329–341.
Article4. Blandizzi C, Viscomi GC, Scarpignato C. Impact of crystal polymorphism on the systemic bioavailability of rifaximin, an antibiotic acting locally in the gastrointestinal tract, in healthy volunteers. Drug Des Devel Ther. 2015; 9:1–11.5. Carrascosa A, De la Peña A, Gutiérrez L, Sumano H. Serum pharmacokinetics and tissue concentrations of a new recrystallized enrofloxacin hydrochloride-dihydrate in hamsters. Turk J Vet Anim Sci. 2015; 39:661–667.
Article6. Carrascosa A, Gutierrez L, De la Peña A, Candanosa IE, Tapia G, Sumano H. Efficacy of a new recrystallized enrofloxacin hydrochloride-dihydrate against leptospirosis in a hamster model. Antimicrob Agents Chemother. 2017; 61:e01285–e01217.
Article7. Censi R, Di Martino P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules. 2015; 20:18759–18776.
Article8. Cester CC, Schneider M, Toutain PL. Comparative kinetics of two orally administered fluoroquinolones in dog: Enrofloxacin versus Marbofloxacin. Rev Med Vet (Toulouse). 1996; 147:703–716.9. Chakravarthy VA, Sailaja BB, Kumar AP. Stability-indicating RP-HPLC method for simultaneous estimation of enrofloxacin and its degradation products in tablet dosage forms. J Anal Methods Chem. 2015; 2015:735145.
Article10. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard-Third Edition. CLSI document M31-A3. Wayne: CLSI;2008.11. Costa E, Lopes AA, Sacramento E, Costa YA, Matos ED, Lopes MB, Bina JC. Penicillin at the late stage of leptospirosis: a randomized controlled trial. Rev Inst Med Trop Sao Paulo. 2003; 45:141–145.
Article12. Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. 2nd ed. Melbourne: Medisci Press;2000.13. Greene CE. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis: Saunders Elsevier;2012.14. Griffith ME, Moon JE, Johnson EN, Clark KP, Hawley JS, Hospenthal DR, Murray CK. Efficacy of fluoroquinolones against Leptospira interrogans in a hamster model. Antimicrob Agents Chemother. 2007; 51:2615–2617.
Article15. Gutierrez L, Miranda-Calderon JE, Garcia-Gutierrez P, Sumano H. Physicochemical characterization and pharmacokinetics in broiler chickens of a new recrystallized enrofloxacin hydrochloride dihydrate. J Vet Pharmacol Ther. 2015; 38:183–189.
Article16. Heinen E. Comparative serum pharmacokinetics of the fluoroquinolones enrofloxacin, difloxacin, marbofloxacin, and orbifloxacin in dogs after single oral administration. J Vet Pharmacol Ther. 2002; 25:1–5.
Article17. Idowu OR, Peggins JO. Simple, rapid determination of enrofloxacin and ciprofloxacin in bovine milk and plasma by high-performance liquid chromatography with fluorescence detection. J Pharm Biomed Anal. 2004; 35:143–153.
Article18. Kim D, Kordick D, Divers T, Chang YF. In vitro susceptibilities of Leptospira spp. and Borrelia burgdorferi isolates to amoxicillin, tilmicosin, and enrofloxacin. J Vet Sci. 2006; 7:355–359.
Article19. Martínez-Cortés I, Gutierrez L, Tapia G, Ocampo L, Sumano H. Serum and milk concentrations of enrofloxacin in cows intramammarily treated with a new enrofloxacin-polymorph. Med Weter. 2016; 72:686–692.
Article20. McKellar QA, Sanchez Bruni SF, Jones DG. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J Vet Pharmacol Ther. 2004; 27:503–514.
Article21. Meinen JB, McClure JT, Rosin E. Pharmacokinetics of enrofloxacin in clinically normal dogs and mice and drug pharmacodynamics in neutropenic mice with Escherichia coli and staphylococcal infections. Am J Vet Res. 1995; 56:1219–1224.22. Miraglia F, Matsuo M, Morais ZM, Dellagostin OA, Seixas FK, Freitas JC, Hartskeerl R, Moreno LZ, Costa BL, Souza GO, Vasconcellos SA, Moreno AM. Molecular characterization, serotyping, and antibiotic susceptibility profile of Leptospira interrogans serovar Copenhageni isolates from Brazil. Diagn Microbiol Infect Dis. 2013; 77:195–199.
Article23. Miranda-Calderón JE, Gutiérrez L, Flores-Alamo M, García-Gutiérrez P, Sumano H. Enrofloxacin hydrochloride dihydrate. Acta Crystallogr Sect E Struct Rep Online. 2014; 70:o468–o469.
Article24. Moon JE, Ellis MW, Griffith ME, Hawley JS, Rivard RG, McCall S, Hospenthal DR, Murray CK. Efficacy of macrolides and telithromycin against leptospirosis in a hamster model. Antimicrob Agents Chemother. 2006; 50:1989–1992.
Article25. Mouton JW, Punt N. Use of the t > MIC to choose between different dosing regimens of beta-lactam antibiotics. J Antimicrob Chemother. 2001; 47:500–501.
Article26. Murray CK, Hospenthal DR. Broth microdilution susceptibility testing for Leptospira spp. Antimicrob Agents Chemother. 2004; 48:1548–1552.
Article27. Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013; 46:245–252.
Article28. Papich MG. Saunders Handbook of Veterinary Drugs. 2nd ed. St. Louis: Saunders Elsevier;2007. p. 236–238.29. Plank R, Dean D. Overview of the epidemiology, microbiology, and pathogenesis of Leptospira spp. in humans. Microbes Infect. 2000; 2:1265–1276.
Article30. Raza K, Kumar P, Ratan S, Malik S, Arora S. Polymorphism: the phenomenon affecting the performance of drugs. SOJ Pharm Pharm Sci. 2014; 1:10.
Article31. Rojas P, Monahan AM, Schuller S, Miller IS, Markey BK, Nally JE. Detection and quantification of leptospires in urine of dogs: a maintenance host for the zoonotic disease leptospirosis. Eur J Clin Microbiol Infect Dis. 2010; 29:1305–1309.
Article32. Schuller S, Francey T, Hartmann K, Hugonnard M, Kohn B, Nally JE, Sykes J. European consensus statement on leptospirosis in dogs and cats. J Small Anim Pract. 2015; 56:159–179.
Article33. Simon Z, Katja B, Darko U, Marjan V, Albin K. Metal cation-fluoroquinolone complexes do not permeate through the intestinal absorption barrier. J Pharm Biomed Anal. 2010; 53:655–659.
Article34. Sumano LH, Gutiérrez OL, Zamora MA. Bioequivalence of four preparations of enrofloxacin in poultry. J Vet Pharmacol Ther. 2001; 24:309–313.
Article35. Sumano LH, Ocampo CL, Gutiérrez OL. Bioequivalence of six generic preparations of enrofloxacin in pigs. Pig J. 2003; 51:64–73.36. Sumano LH, Ocampo CL, Gutiérrez OL. Non-bioequivalence of various trademarks of enrofloxacin and Baytril in cows. Dtsch Tierarztl Wochenschr. 2001; 108:311–314.37. Sykes JE, Hartmann K, Lunn KF, Moore GE, Stoddard RA, Goldstein RE. 2010 ACVIM small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J Vet Intern Med. 2011; 25:1–13.
Article38. Trouchon T, Lefebvre S. A review of enrofloxacin for veterinary use. Open J Vet Med. 2016; 6:40–58.
Article39. Variankaval N, Cote AS, Doherty MF. From form to function: crystallization of active pharmaceutical ingredients. Am Inst Chem Engins J. 2008; 54:1682–1688.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful treatment of recurrent subclinical mastitis in cows caused by enrofloxacin resistant bacteria by means of the sequential intramammary infusion of enrofloxacin HCl-2H 2 O and ceftiofur HCl: a clinical trial
- Monte Carlo Simulation Codes for Nuclear Medicine Imaging
- Intramammary preparation of enrofloxacin hydrochloride-dihydrate for bovine mastitis (biofilm-forming Staphylococcus aureus)
- In vitro susceptibilities of Leptospira spp. and Borrelia burgdorferi isolates to amoxicillin, tilmicosin, and enrofloxacin
- Verification of the PMCEPT Monte Carlo dose Calculation Code for Simulations in Medical Physics