Ann Dermatol.
2018 Oct;30(5):556-561. 10.5021/ad.2018.30.5.556.
Sensitivity and Usefulness of VE1 Immunohistochemical Staining in Acral Melanomas with BRAF Mutation
- Affiliations
-
- 1Department of Dermatology, Chonnam National University Medical School, Gwangju, Korea. sjyun@chonnam.ac.kr
- 2Department of Pathology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2419745
- DOI: http://doi.org/10.5021/ad.2018.30.5.556
Abstract
- BACKGROUND
Acral melanomas are known to have a low frequency of BRAF mutation, in contrary to higher KIT mutation. Recently, VE1 immunostaining was reported to have a good correlation with BRAF mutation status.
OBJECTIVE
We aimed to evaluate the clinicopathological features of BRAF-mutated acral melanomas and validate the correlation of the VE1 immunohistochemical stains in those cases.
METHODS
The clinical features (age, sex, anatomical site), and histopathological characteristics of 41 patients with acral melanoma were evaluated. We performed a next-generation sequencing to detect BRAF mutation status. We also determined the correlation of VE1 immunohistochemical staining with BRAF mutation status.
RESULTS
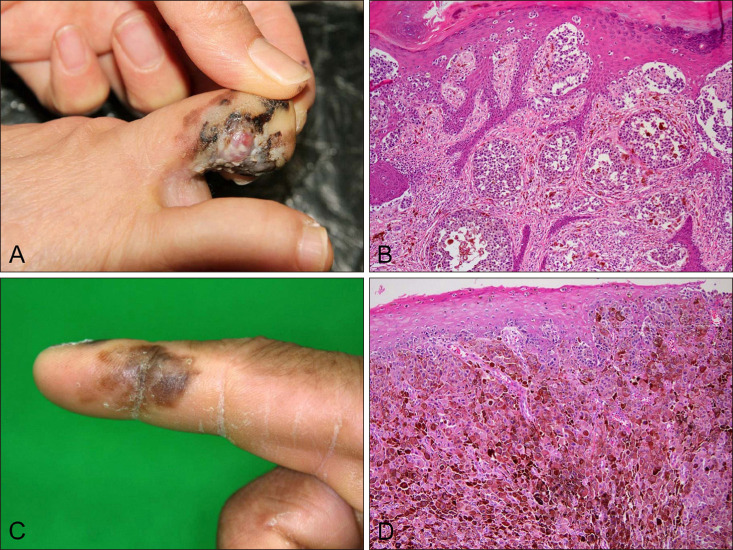
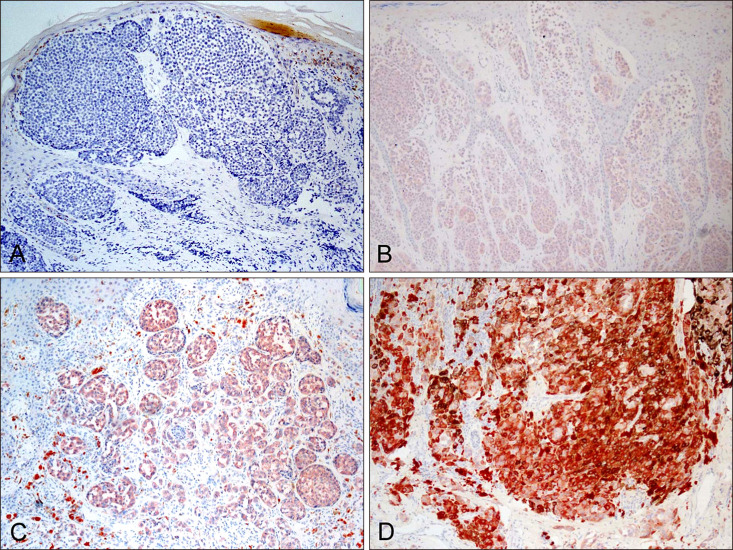
Among 19 acral melanomas with BRAF mutation, common histopathological subtype was acral lentiginous melanoma (8/19, 42%) and nodular melanoma (8/19, 42%) and superficial spreading melanoma (3/19, 16%) followed. VE1 immunostaining results were positive in all 15 cases with BRAF V600E mutation (sensitivity 100%), and negative in 4 cases of BRAF non-V600E mutation. However, VE1 immunostaining was negative in all 22 patients with BRAF wild-type.
CONCLUSION
VE1 immunostaining had a good correlation with BRAF V600E mutation status.
Keyword
Figure
Reference
-
1. Kim SY, Yun SJ. Cutaneous melanoma in Asians. Chonnam Med J. 2016; 52:185–193. PMID: 27689028.
Article2. National Cancer Institute. FDA approval for vemurafenib [Internet]. Rockville, MD: National Cancer Institute [cited 2011 Sep 9];Available from: http://cancer.gov/about-cancer/treatment/drugs/fda-vemurafenib.3. Wilson MA, Nathanson KL. Molecular testing in melanoma. Cancer J. 2012; 18:117–123. PMID: 22453011.
Article4. National Cancer Institute. FDA approval for Dabrafenib [Internet]. Rockville, MD: National Cancer Institute;cited 2013 Jun 21. Available from: https://www.cancer.gov/aboutcancer/treatment/drugs/dabrafenib.5. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417:949–954. PMID: 12068308.
Article6. Rubinstein JC, Sznol M, Pavlick AC, Arian S, Cheng E, Bacchiocchi A, et al. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010; 8:67. PMID: 20630094.
Article7. Edlundh-Rose E, Egyházi S, Omholt K, Månsson-Brahme E, Platz A, Hansson J, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res. 2006; 16:471–478. PMID: 17119447.
Article8. Saldanha G, Potter L, Daforno P, Pringle JH. Cutaneous melanoma subtypes show different BRAF and NRAS mutation frequencies. Clin Cancer Res. 2006; 12:4499–4505. PMID: 16899595.
Article9. Lang J, MacKie RM. Prevalence of exon 15 BRAF mutations in primary melanoma of the superficial spreading, nodular, acral, and lentigo maligna subtypes. J Invest Dermatol. 2005; 125:575–579. PMID: 16117801.
Article10. Jin SA, Chun SM, Choi YD, Kweon SS, Jung ST, Shim HJ, et al. BRAF mutations and KIT aberrations and their clinicopathological correlation in 202 Korean melanomas. J Invest Dermatol. 2013; 133:579–582. PMID: 23014346.
Article11. Hong JW, Lee S, Kim DC, Kim KH, Song KH. Prognostic and clinicopathologic associations of BRAF mutation in primary acral lentiginous melanoma in Korean patients: a preliminary study. Ann Dermatol. 2014; 26:195–202. PMID: 24882974.12. Pearlstein MV, Zedek DC, Ollila DW, Treece A, Gulley ML, Groben PA, et al. Validation of the VE1 immunostain for the BRAF V600E mutation in melanoma. J Cutan Pathol. 2014; 41:724–732. PMID: 24917033.13. Marin C, Beauchet A, Capper D, Zimmermann U, Julié C, Ilie M, et al. Detection of BRAF p.V600E mutations in melanoma by immunohistochemistry has a good interobserver reproducibility. Arch Pathol Lab Med. 2014; 138:71–75. PMID: 23651150.14. Capper D, Preusser M, Habel A, Sahm F, Ackermann U, Schindler G, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011; 122:11–19. PMID: 21638088.
Article15. Long GV, Wilmott JS, Capper D, Preusser M, Zhang YE, Thompson JF, et al. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol. 2013; 37:61–65. PMID: 23026937.
Article16. Manfredi L, Meyer N, Tournier E, Grand D, Uro-Coste E, Rochaix P, et al. Highly concordant results between immunohistochemistry and molecular testing of mutated V600E BRAF in primary and metastatic melanoma. Acta Derm Venereol. 2016; 96:630–634. PMID: 26695089.
Article17. Capper D, Berghoff AS, Magerle M, Ilhan A, Wöhrer A, Hackl M, et al. Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol. 2012; 123:223–233. PMID: 22012135.
Article18. Yaman B, Kandiloğlu G, Akalin T. BRAF-V600 Mutation heterogeneity in primary and metastatic melanoma: a study with pyrosequencing and immunohistochemistry. Am J Dermatopathol. 2016; 38:113–120. PMID: 26630683.19. Li H, Drubin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009; 25:1754–1760. PMID: 19451168.
Article20. DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2010; 43:491–498.
Article21. Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, et al. From FastQ data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013; 43:11.10.1–11.10.33. PMID: 25431634.22. Wilm A, Aw PP, Bertrand D, Yeo GH, Ong SH, Wong CH, et al. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res. 2012; 40:11189–11201. PMID: 23066108.
Article23. Wei Z, Wang W, Hu P, Lyon GJ, Hakonarson H. SNVer: a statistical tool for variant calling in analysis of pooled or individual next-generation sequencing data. Nucleic Acids Res. 2011; 39:e132. PMID: 21813454.
Article24. Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017; 545:175–180. PMID: 28467829.
Article25. Wu X, Yan J, Dai J, Ma M, Tang H, Yu J, et al. Mutations in BRAF codons 594 and 596 predict good prognosis in melanoma. Oncol Lett. 2017; 14:3601–3605. PMID: 28927118.
Article26. Boursault L, Haddad V, Vergier B, Cappellen D, Verdon S, Bellocq JP, et al. Tumor homogeneity between primary and metastatic sites for BRAF status in metastatic melanoma determined by immunohistochemical and molecular testing. PLoS One. 2013; 8:e70826. PMID: 23976959.
Article27. Rapisuwon S, Busam KJ, Parks K, Chapman PB, Lee E, Atkins MB. Discordance between cobas BRAF V600 testing and VE1 immunohistochemistry in a melanoma patient with bone marrow metastases. Am J Dermatopathol. 2016; 38:687–689. PMID: 27541170.
Article28. Heinzerling L, Kühnapfel S, Meckbach D, Baiter M, Kaempgen E, Keikavoussi P, et al. Rare BRAF mutations in melanoma patients: implications for molecular testing in clinical practice. Br J Cancer. 2013; 108:2164–2171. PMID: 23579220.
Article29. Ihle MA, Fassunke J, König K, Grünewald I, Schlaak M, Kreuzberg N, et al. Comparison of high resolution melting analysis, pyrosequencing, next generation sequencing and immunohistochemistry to conventional Sanger sequencing for the detection of p.V600E and non-p.V600E BRAF mutations. BMC Cancer. 2014; 14:13. PMID: 24410877.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Utility of BRAF VE1 Immunohistochemistry as a Screening Tool for Colorectal Cancer Harboring BRAF V600E Mutation
- Usefulness of BRAF VE1 immunohistochemistry in non–small cell lung cancers: a multi-institutional study by 15 pathologists in Korea
- Prognostic and Clinicopathologic Associations of BRAF Mutation in Primary Acral Lentiginous Melanoma in Korean Patients: A Preliminary Study
- Selection Strategies and Practical Application of BRAF V600E-Mutated Non–Small Cell Lung Carcinoma
- Evaluation of the VE1 Antibody in Thyroid Cytology Using Ex Vivo Papillary Thyroid Carcinoma Specimens