World J Mens Health.
2018 May;36(2):139-146. 10.5534/wjmh.170005.
Silencing Histone Deacetylase 7 Alleviates Transforming Growth Factor-β1-Induced Profibrotic Responses in Fibroblasts Derived from Peyronie's Plaque
- Affiliations
-
- 1National Research Center for Sexual Medicine, Inha University School of Medicine, Incheon, Korea. jksuh@inha.ac.kr, rjk0929@inha.ac.kr
- 2Department of Urology, Inha University School of Medicine, Incheon, Korea.
- 3Inha Research Institute for Medical Sciences, Inha University School of Medicine, Incheon, Korea.
- KMID: 2419312
- DOI: http://doi.org/10.5534/wjmh.170005
Abstract
- PURPOSE
Epigenetic modifications, such as histone acetylation/deacetylation and DNA methylation, play a crucial role in the pathogenesis of inflammatory disorders and fibrotic diseases. The aim of this study was to study the differential gene expression of histone deacetylases (HDACs) in fibroblasts isolated from plaque tissue of Peyronie's disease (PD) or normal tunica albuginea (TA) and to examine the anti-fibrotic effect of small interfering RNA (siRNA)-mediated silencing of HDAC7 in fibroblasts derived from human PD plaque.
MATERIALS AND METHODS
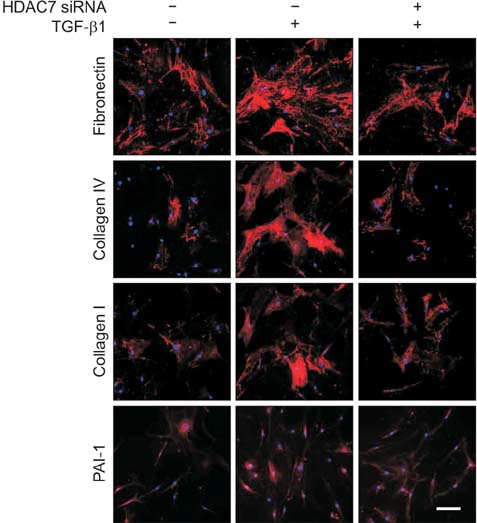
For differential gene expression study, we performed reverse-transcriptase polymerase chain reaction for HDAC isoforms (1-11) in fibroblasts isolated from PD plaque or normal TA. Fibroblasts isolated from PD plaque were pretreated with HDAC7 siRNA (100 pmol) and then stimulated with transforming growth factor-β1 (TGF-β1, 10 ng/mL). Protein was extracted from treated fibroblasts for Western blotting. We also performed immunocytochemistry to detect the expression of extracellular matrix proteins and to examine the effect of HDAC2 siRNA on the TGF-β1-induced nuclear translocation of Smad2/3 and myofibroblastic differentiation.
RESULTS
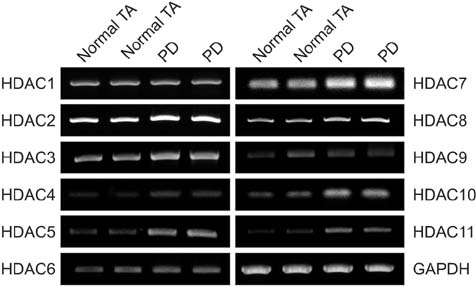
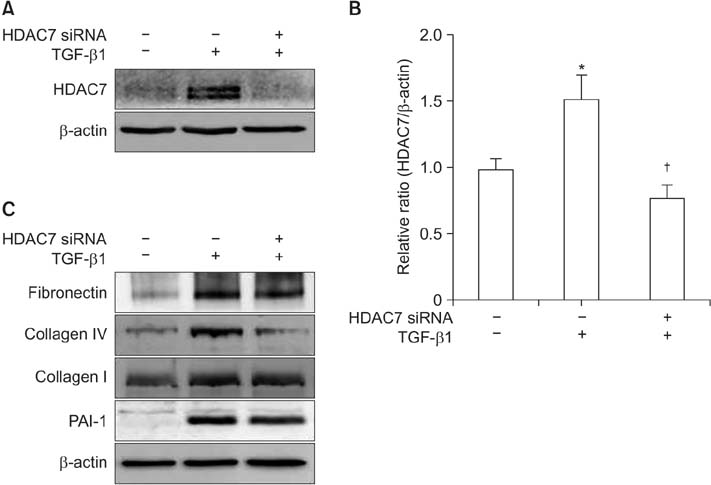
The mRNA expression of HDAC2, 3, 4, 5, 7, 8, 10, and 11 was higher in fibroblasts isolated from PD plaque than in fibroblasts isolated from normal TA tissue. Knockdown of HDAC7 in PD fibroblasts inhibited TGF-β1-induced nuclear shuttle of Smad2 and Smad3, transdifferentiation of fibroblasts into myofibroblasts, and abrogated TGF-β1-induced production of extracellular matrix protein.
CONCLUSIONS
These findings suggest that specific inhibition of HDAC7 with RNA interference may represent a promising epigenetic therapy for PD.
Keyword
MeSH Terms
-
Blotting, Western
DNA Methylation
Epigenomics
Extracellular Matrix
Extracellular Matrix Proteins
Fibroblasts*
Fibrosis
Gene Expression
Histone Deacetylases*
Histones*
Humans
Immunohistochemistry
Male
Myofibroblasts
Penile Induration
Polymerase Chain Reaction
Protein Isoforms
RNA Interference
RNA, Messenger
RNA, Small Interfering
Transforming Growth Factors
Extracellular Matrix Proteins
Histone Deacetylases
Histones
Protein Isoforms
RNA, Messenger
RNA, Small Interfering
Transforming Growth Factors
Figure
Cited by 1 articles
-
Extracorporeal Shock Wave Therapy in Peyronie's Disease: Clinical Efficacy and Safety from a Single-Arm Observational Study
Marina di Mauro, Giorgio Ivan Russo, Pier Andrea Della Camera, Fabrizio di Maida, Gianmartin Cito, Nicola Mondaini, Marco Capece, Marco Falcone, Francesco Sessa, Andrea Mari, Riccardo Campi, Carlotta Sabini, Sergio Serni, Mauro Gacci, Andrea Minervini, Marco Carini, Sebastiano Cimino, Girolamo Morelli, Andrea Cocci
World J Mens Health. 2019;37(3):339-346. doi: 10.5534/wjmh.180100.
Reference
-
1. Gholami SS, Gonzalez-Cadavid NF, Lin CS, Rajfer J, Lue TF. Peyronie's disease: a review. J Urol. 2003; 169:1234–1241.
Article2. Hellstrom WJ, Bivalacqua TJ. Peyronie's disease: etiology, medical, and surgical therapy. J Androl. 2000; 21:347–354.3. Devine CJ Jr, Somers KD, Jordan SG, Schlossberg SM. Proposal: trauma as the cause of the Peyronie's lesion. J Urol. 1997; 157:285–290.
Article4. Jarow JP, Lowe FC. Penile trauma: an etiologic factor in Peyronie's disease and erectile dysfunction. J Urol. 1997; 158:1388–1390.
Article5. Gabrielson AT, Alzweri LM, Hellstrom WJ. Collagenase clostridium histolyticum in the treatment of Peyronie's disease: review of a minimally invasive treatment option. World J Mens Health. 2017; 35:134–145.
Article6. Joice GA, Burnett AL. Nonsurgical interventions for Peyronie's disease: update as of 2016. World J Mens Health. 2016; 34:65–72.
Article7. Segal RL, Burnett AL. Surgical management for Peyronie's disease. World J Mens Health. 2013; 31:1–11.
Article8. McKinsey TA. Targeting inflammation in heart failure with histone deacetylase inhibitors. Mol Med. 2011; 17:434–441.
Article9. Pang M, Zhuang S. Histone deacetylase: a potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther. 2010; 335:266–272.
Article10. Huber LC, Distler JH, Moritz F, Hemmatazad H, Hauser T, Michel BA, et al. Trichostatin A prevents the accumulation of extracellular matrix in a mouse model of bleomycin-induced skin fibrosis. Arthritis Rheum. 2007; 56:2755–2764.
Article11. Hemmatazad H, Rodrigues HM, Maurer B, Brentano F, Pileckyte M, Distler JH, et al. Histone deacetylase 7, a potential target for the antifibrotic treatment of systemic sclerosis. Arthritis Rheum. 2009; 60:1519–1529.
Article12. Piao S, Choi MJ, Tumurbaatar M, Kim WJ, Jin HR, Shin SH, et al. Transforming growth factor (TGF)-β type I receptor kinase (ALK5) inhibitor alleviates profibrotic TGF-β1 responses in fibroblasts derived from Peyronie's plaque. J Sex Med. 2010; 7:3385–3395.
Article13. Ryu JK, Kim WJ, Choi MJ, Park JM, Song KM, Kwon MH, et al. Inhibition of histone deacetylase 2 mitigates profibrotic TGF-β1 responses in fibroblasts derived from Peyronie's plaque. Asian J Androl. 2013; 15:640–645.
Article14. Hoodless PA, Haerry T, Abdollah S, Stapleton M, O'Connor MB, Attisano L, et al. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996; 85:489–500.
Article15. Korfei M, Skwarna S, Henneke I, MacKenzie B, Klymenko O, Saito S, et al. Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax. 2015; 70:1022–1032.
Article16. Hutt DM, Herman D, Rodrigues AP, Noel S, Pilewski JM, Matteson J, et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol. 2010; 6:25–33.
Article17. Pannem RR, Dorn C, Hellerbrand C, Massoumi R. Cylindromatosis gene CYLD regulates hepatocyte growth factor expression in hepatic stellate cells through interaction with histone deacetylase 7. Hepatology. 2014; 60:1066–1081.
Article18. Glenisson W, Castronovo V, Waltregny D. Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim Biophys Acta. 2007; 1773:1572–1582.19. Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009; 297:L864–L870.20. Noh H, Oh EY, Seo JY, Yu MR, Kim YO, Ha H, et al. Histone deacetylase-2 is a key regulator of diabetes- and transforming growth factor-beta1-induced renal injury. Am J Physiol Renal Physiol. 2009; 297:F729–F739.21. Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, et al. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2009; 297:F996–F1005.
Article22. El-Sakka AI, Hassoba HM, Pillarisetty RJ, Dahiya R, Lue TF. Peyronie's disease is associated with an increase in transforming growth factor-beta protein expression. J Urol. 1997; 158:1391–1394.
Article23. Haag SM, Hauck EW, Szardening-Kirchner C, Diemer T, Cha ES, Weidner W, et al. Alterations in the transforming growth factor (TGF)-beta pathway as a potential factor in the pathogenesis of Peyronie's disease. Eur Urol. 2007; 51:255–261.24. Kwon KD, Choi MJ, Park JM, Song KM, Kwon MH, Batbold D, et al. Silencing histone deacetylase 2 using small hairpin RNA induces regression of fibrotic plaque in a rat model of Peyronie's disease. BJU Int. 2014; 114:926–936.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activin Receptor-Like Kinase 5 Inhibitor Attenuates Fibrosis in Fibroblasts Derived from Peyronie's Plaque
- Inhibitors of DNA methylation support TGF-β1-induced IL11 expression in gingival fibroblasts
- Effect of Manuka Honey on Transforming Growth Factor Beta-1-Induced Extracelluar Matrix Production in Nasal Polyp Derived Fibroblasts
- Role of the transforming growth factor (TGF)-β1 and TGF-β1 signaling pathway on the pathophysiology of respiratory pneumococcal infections
- A Study on the Proliferation and the Collagen Synthesis of the Fibroblasts Cultured from Normal Skin and Hypertrophic Scar