Yonsei Med J.
2017 May;58(3):505-513. 10.3349/ymj.2017.58.3.505.
Cyclized Oligopeptide Targeting LRP5/6-DKK1 Interaction Reduces the Growth of Tumor Burden in a Multiple Myeloma Mouse Model
- Affiliations
-
- 1Brain Korea 21 PLUS Project for Medical Science, Yonsei University, Seoul, Korea. lsk@yuhs.ac
- 2Institute of Biomedical Sciences, Yonsei University, Seoul, Korea.
- 3Division of Endocrinology and Endocrine Research Institute, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Biochemistry, Yonsei University, Seoul, Korea.
- 5Centre for Immune Regulation, Institute of Immunology, University of Oslo and Oslo University Hospital, Oslo, Norway.
- KMID: 2419106
- DOI: http://doi.org/10.3349/ymj.2017.58.3.505
Abstract
- PURPOSE
Dickkopf 1 (DKK1) has been extensively investigated in mouse models of multiple myeloma, which results in osteolytic bone lesions. Elevated DKK1 levels in bone marrow plasma and serum inhibit the differentiation of osteoblast precursors. Present pharmaceutical approaches to target bone lesions are limited to antiresorptive agents. In this study, we developed a cyclized oligopeptide against DKK1-low density lipoprotein receptor-related protein (LRP) 5/6 interaction and tested the effects of the oligopeptide on tumor burden.
MATERIALS AND METHODS
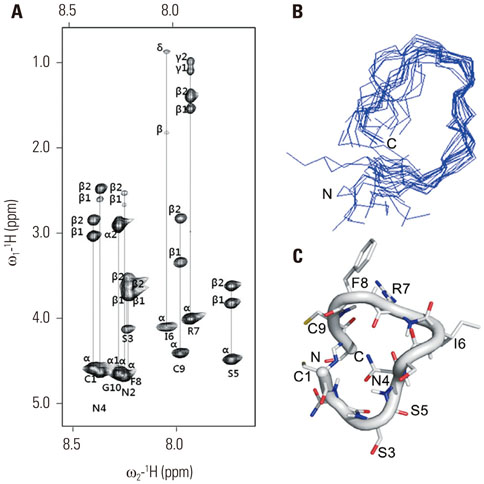
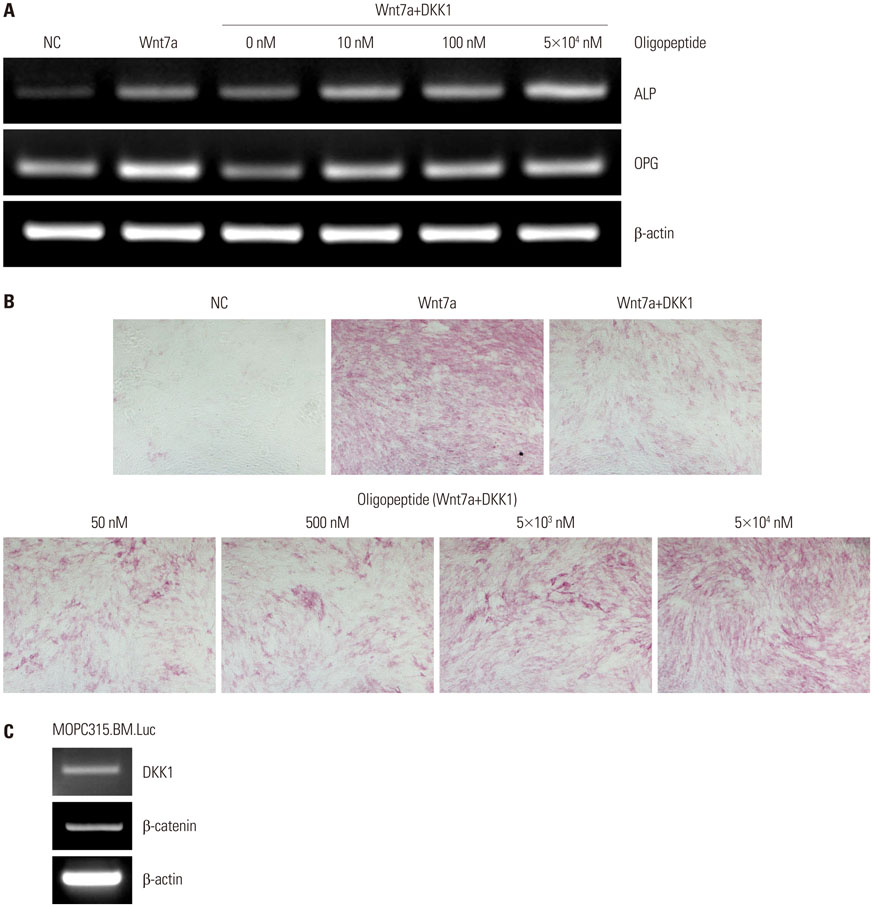
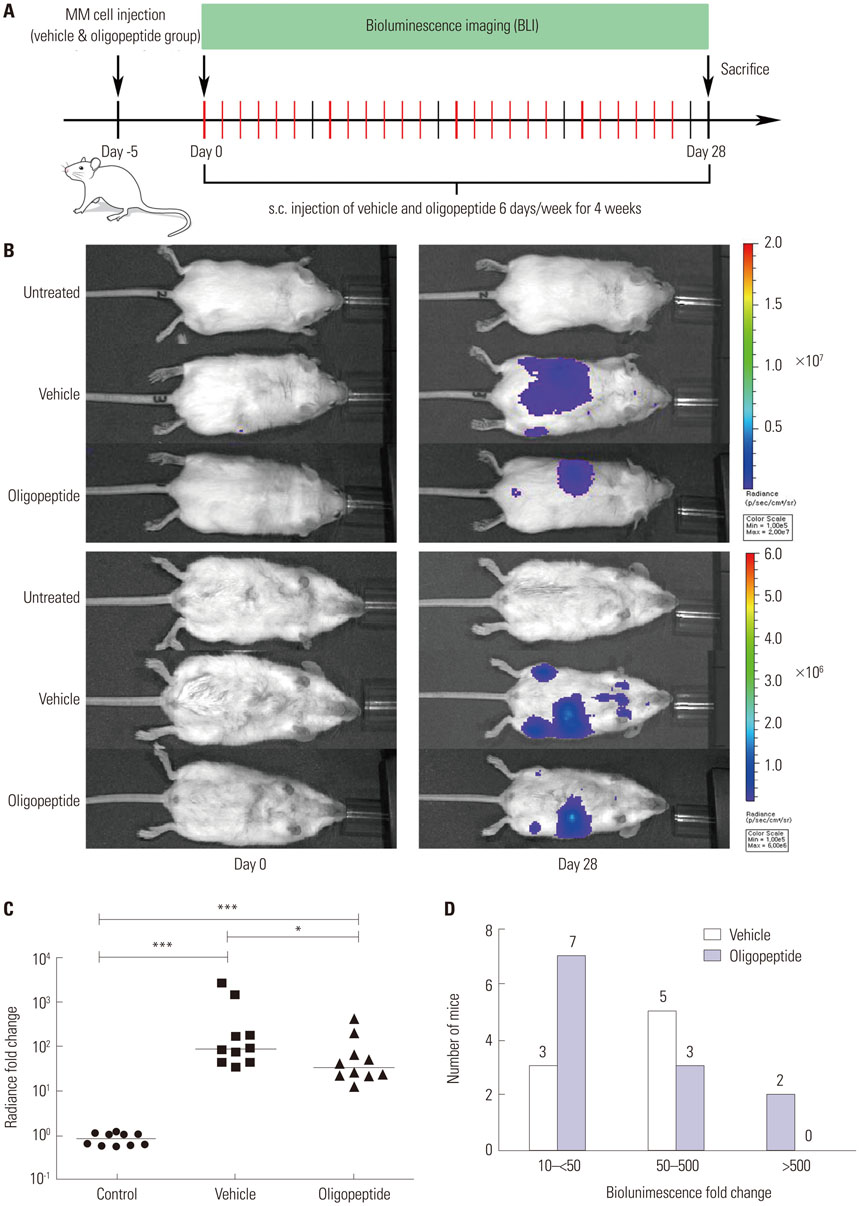
A cyclized oligopeptide based on DKK1-LRP5/6 interactions was synthesized chemically, and its nuclear magnetic resonance structure was assessed. Luciferase reporter assay and mRNA expressions of osteoblast markers were evaluated after oligopeptide treatment. MOPC315.BM.Luc cells were injected into the tail vein of mice, after which cyclized oligopeptide was delivered subcutaneously 6 days a week for 4 weeks.
RESULTS
The cyclized oligopeptide containing NXI motif bound to the E1 domain of LRP5/6 effectively on surface plasmon resonance analysis. It abrogated the Wnt-β-catenin signaling inhibited by DKK1, but not by sclerostin, dose dependently. RT-PCR and alkaline phosphatase staining showed increased expressions of osteoblast markers according to the treatment concentrations. Bioluminescence images showed that the treatment of cyclized oligopeptide reduced tumor burden more in oligopeptide treated group than in the vehicle group.
CONCLUSION
The cyclized oligopeptide reported here may be another option for the treatment of tumor burden in multiple myeloma.
Keyword
MeSH Terms
-
Animals
Bone Marrow/*metabolism
Cell Differentiation/drug effects
Cell Line, Tumor
Disease Models, Animal
Intercellular Signaling Peptides and Proteins/*metabolism
Mice
Multiple Myeloma/*complications/pathology/*physiopathology
Oligopeptides/*pharmacology
Osteoblasts/*drug effects/pathology
Signal Transduction
Tumor Burden/*drug effects
Wnt Proteins/metabolism
beta Catenin
Intercellular Signaling Peptides and Proteins
Oligopeptides
Wnt Proteins
beta Catenin
Figure
Reference
-
1. Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009; 374:324–339.
Article2. Balakumaran A, Robey PG, Fedarko N, Landgren O. Bone marrow microenvironment in myelomagenesis: its potential role in early diagnosis. Expert Rev Mol Diagn. 2010; 10:465–480.
Article3. Ballester OF, Moscinski LC, Lyman GH, Chaney JV, Saba HI, Spiers AS, et al. High levels of interleukin-6 are associated with low tumor burden and low growth fraction in multiple myeloma. Blood. 1994; 83:1903–1908.
Article4. Lee JW, Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD, et al. IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood. 2004; 103:2308–2315.
Article5. Heider U, Zavrski I, Jakob C, Bängeroth K, Fleissner C, Langelotz C, et al. Expression of receptor activator of NF-kappaB ligand (RANKL) mRNA in human multiple myeloma cells. J Cancer Res Clin Oncol. 2004; 130:469–474.6. Sati HI, Greaves M, Apperley JF, Russell RG, Croucher PI. Expression of interleukin-1beta and tumour necrosis factor-alpha in plasma cells from patients with multiple myeloma. Br J Haematol. 1999; 104:350–357.
Article7. Choi SJ, Cruz JC, Craig F, Chung H, Devlin RD, Roodman GD, et al. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000; 96:671–675.
Article8. Mukai T, Otsuka F, Otani H, Yamashita M, Takasugi K, Inagaki K, et al. TNF-alpha inhibits BMP-induced osteoblast differentiation through activating SAPK/JNK signaling. Biochem Biophys Res Commun. 2007; 356:1004–1010.
Article9. Ehrlich LA, Chung HY, Ghobrial I, Choi SJ, Morandi F, Colla S, et al. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood. 2005; 106:1407–1414.
Article10. Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004; 93:1210–1230.
Article11. Kim JH, Liu X, Wang J, Chen X, Zhang H, Kim SH, et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther Adv Musculoskelet Dis. 2013; 5:13–31.
Article12. Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009; 24:578–588.
Article13. Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, et al. Isolation and biochemical characterization of the human Dkk1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999; 274:19465–19472.
Article14. Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG, et al. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am. 2007; 21:1007–1034.
Article15. Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, et al. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008; 112:196–207.
Article16. Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003; 349:2483–2494.
Article17. Rachner TD, Hadji P, Hofbauer LC. Novel therapies in benign and malignant bone diseases. Pharmacol Ther. 2012; 134:338–344.
Article18. Rachner TD, Göbel A, Benad-Mehner P, Hofbauer LC, Rauner M. Dickkopf-1 as a mediator and novel target in malignant bone disease. Cancer Lett. 2014; 346:172–177.
Article19. Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007; 109:2106–2111.
Article20. Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009; 114:371–379.
Article21. Hofgaard PO, Jodal HC, Bommert K, Huard B, Caers J, Carlsen H, et al. A novel mouse model for multiple myeloma (MOPC315.BM) that allows noninvasive spatiotemporal detection of osteolytic disease. PLoS One. 2012; 7:e51892.
Article22. Davis DG, Bax A. Assignment of complex proton NMR spectra via two-dimensional homonuclear Hartnabb-Hahn spectroscopy. J Am Chem Soc. 1985; 107:2820–2821.
Article23. Jeener J, Meier BH, Bachman P, Ernst RR. Investigation of exchange processes by two-dimensional NMR spectroscopy. J Chem Phys. 1979; 71:4546–4553.
Article24. Koradi R, Billeter M, Wüthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph. 1996; 14:51–55.
Article25. Jami A, Gadi J, Lee MJ, Kim EJ, Lee MJ, Jung HS, et al. Pax6 expressed in osteocytes inhibits canonical Wnt signaling. Mol Cells. 2013; 35:305–312.
Article26. Zhou F, Meng S, Song H, Claret FX. Dickkopf-1 is a key regulator of myeloma bone disease: opportunities and challenges for therapeutic intervention. Blood Rev. 2013; 27:261–267.
Article27. D'Amico L, Capietto AH, Zamani A, Faccio R, Bumpass D. Dickkopf-realtaed protein 1 (Dkk1) exerts immune suppressive effects in cancer by regulating expansion and function of myeloid derived suppressor cells. Seattle: Paper presented at Annual Meeting of the American Society for Bone and Mineral Research;2015.28. Wong D, Winter O, Hartig C, Siebels S, Szyska M, Tiburzy B, et al. Eosinophils and megakaryocytes support the early growth of murine MOPC315 myeloma cells in their bone marrow niches. PLoS One. 2014; 9:e109018.
Article29. Smadja DM, d'Audigier C, Weiswald LB, Badoual C, Dangles-Marie V, Mauge L, et al. The Wnt antagonist Dickkopf-1 increases endothelial progenitor cell angiogenic potential. Arterioscler Thromb Vasc Biol. 2010; 30:2544–2552.
Article30. Yata K, Yaccoby S. The SCID-rab model: a novel in vivo system for primary human myeloma demonstrating growth of CD138-expressing malignant cells. Leukemia. 2004; 18:1891–1897.
Article31. Bisping G, Leo R, Wenning D, Dankbar B, Padró T, Kropff M, et al. Paracrine interactions of basic fibroblast growth factor and interleukin-6 in multiple myeloma. Blood. 2003; 101:2775–2783.
Article