Korean J Physiol Pharmacol.
2018 Sep;22(5):577-584. 10.4196/kjpp.2018.22.5.577.
The change of signaling pathway on the electrical stimulated contraction in streptozotocin-induced bladder dysfunction of rats
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 06974, Korea. udsohn@cau.ac.kr
- 2Department of Pharmaceutical Engineering, College of Convergence Science and Technology, Jung Won University, Goesan 28054, Korea.
- 3Pharmaceutical Formulation Design Laboratory, College of Pharmacy, Chung-Ang University , Seoul 06974, Korea.
- 4Department of Pharmacology, School of Pharmacy, Sungkyunkwan University, Suwon 16419, Korea.
- KMID: 2419007
- DOI: http://doi.org/10.4196/kjpp.2018.22.5.577
Abstract
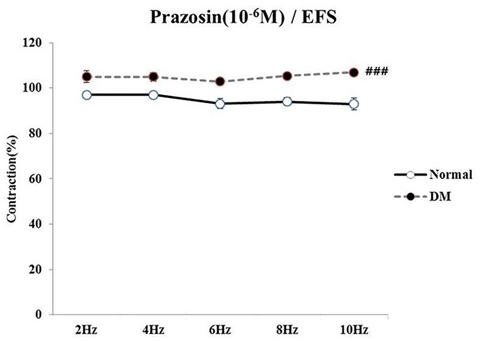
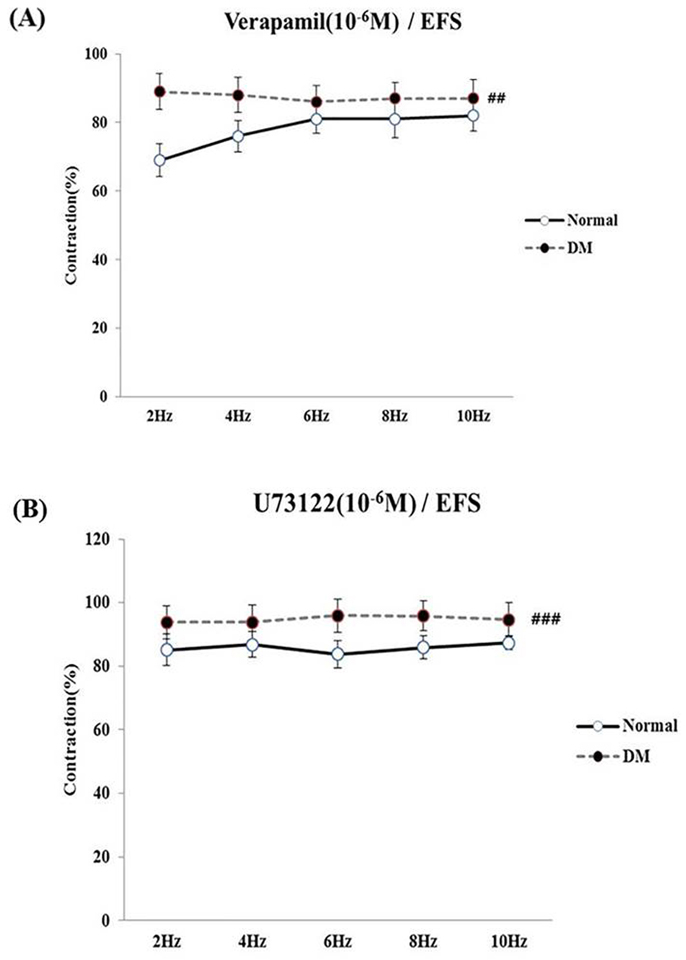
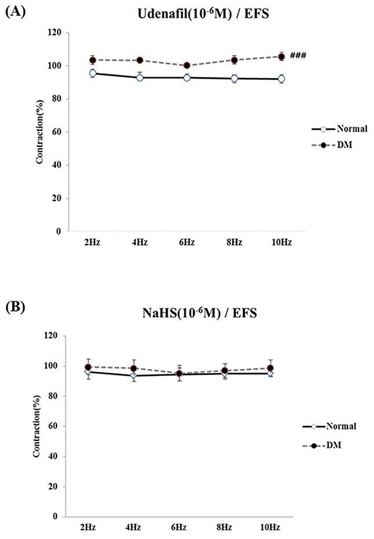
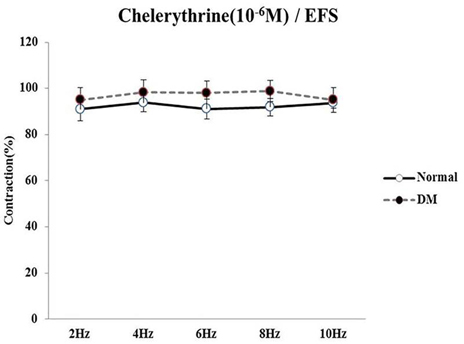
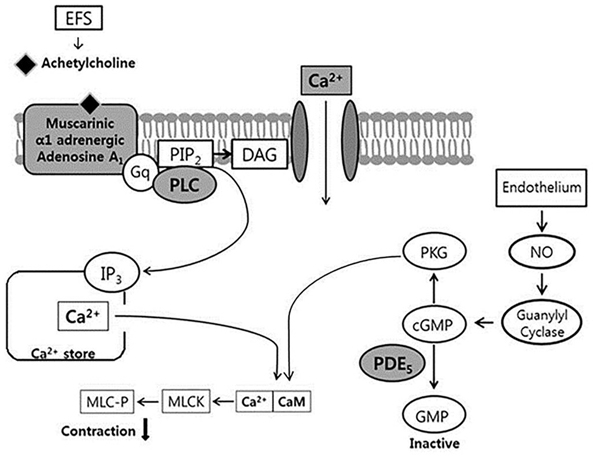
- Bladder dysfunction is a common complication of diabetes mellitus (DM). However, there have been a few studies evaluating bladder smooth muscle contraction in DM in the presence of pharmacological inhibitors. In the present study, we compared the contractility of bladder smooth muscle from normal rats and DM rats. Furthermore, we utilized pharmacological inhibitors to delineate the mechanisms underlying bladder muscle differences between normal and DM rats. DM was established in 14 days after using a single injection of streptozotocin (65 mg/kg, intraperitoneal) in Sprague-Dawley rats. Bladder smooth muscle contraction was induced electrically using electrical field stimulation consisting of pulse trains at an amplitude of 40 V and pulse duration of 1 ms at frequencies of 2-10 Hz. In this study, the pharmacological inhibitors atropine (muscarinic receptor antagonist), U73122 (phospholipase C inhibitor), DPCPX (adenosine Aâ‚ receptor antagonist), udenafil (PDE5 inhibitor), prazosin (αâ‚-receptor antagonist), verapamil (calcium channel blocker), and chelerythrine (protein kinase C inhibitor) were used to pretreat bladder smooth muscles. It was found that the contractility of bladder smooth muscles from DM rats was lower than that of normal rats. In addition, there were significant differences in percent change of contractility between normal and DM rats following pretreatment with prazosin, udenafil, verapamil, and U73122. In conclusion, we suggest that the decreased bladder muscle contractility in DM rats was a result of perturbations in PLC/IP₃-mediated intracellular Ca²âº release and PDE5 activity.
Keyword
MeSH Terms
Figure
Reference
-
1. Pecoits-Filho R, Abensur H, Betônico CC, Machado AD, Parente EB, Queiroz M, Salles JE, Titan S, Vencio S. Interactions between kidney disease and diabetes: dangerous liaisons. Diabetol Metab Syndr. 2016; 8:50.
Article2. Weissman AJ. Intensive diabetes treatment and cardiovascular disease. N Engl J Med. 2006; 354:1751–1752. author reply 1751-1752.
Article3. Amaral N, Okonko DO. Metabolic abnormalities of the heart in type II diabetes. Diab Vasc Dis Res. 2015; 12:239–248.
Article4. Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014; 64:510–533.
Article5. Van Den Eeden SK, Sarma AV, Rutledge BN, Cleary PA, Kusek JW, Nyberg LM, McVary KT, Wessells H. Diabetes Control and Complications Trial/Epidemiology of Diabetes Research Group. Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care. 2009; 32:664–670.
Article6. Ioanid CP, Noica N, Pop T. Incidence and diagnostic aspects of the bladder disorders in diabetics. Eur Urol. 1981; 7:211–214.
Article7. Bradley WE. Diagnosis of urinary bladder dysfunction in diabetes mellitus. Ann Intern Med. 1980; 92:323–326.
Article8. Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995; 153:342–344.
Article9. Todd JK, Mack AJ. A study of human bladder detrusor muscle. Br J Urol. 1969; 41:448–454.
Article10. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004; 84:935–986.
Article11. Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995; 273:959–966.12. Park SY, Je HD, Shim JH, Sohn UD. Characteristics of spontaneous contraction in the circular smooth muscles of cat ileum. Arch Pharm Res. 2010; 33:159–165.
Article13. Sjögren C, Andersson KE, Husted S, Mattiasson A, Moller-Madsen B. Atropine resistance of transmurally stimulated isolated human bladder muscle. J Urol. 1982; 128:1368–1371.
Article14. Min CH, Min YS, Lee SJ, Sohn UD. The comparative effects of aminoglycoside antibiotics and muscle relaxants on electrical field stimulation response in rat bladder smooth muscle. Arch Pharm Res. 2016; 39:863–870.
Article15. Min CH, Wang Y, Bae J, Han JH, Sohn UD. The inhibitory mechanism of gentamicin on electrical field stimulation response in rat bladder smooth muscle. Korean J Physiol Pharmacol. 2015; 19:473–478.
Article16. Braverman AS, Tibb AS, Ruggieri MR Sr. M2 and M3 muscarinic receptor activation of urinary bladder contractile signal transduction. I. Normal rat bladder. J Pharmacol Exp Ther. 2006; 316:869–874.
Article17. Kullmann FA, Downs TR, Artim DE, Limberg BJ, Shah M, Contract D, de Groat WC, Rosenbaum JS. Urothelial beta-3 adrenergic receptors in the rat bladder. Neurourol Urodyn. 2011; 30:144–150.
Article18. Vesela R, Aronsson P, Tobin G. Functional and morphological examinations of P1A1 purinoceptors in the normal and inflamed urinary bladder of the rat. Auton Neurosci. 2011; 159:26–31.
Article19. Johansson R, Pandita RK, Poljakovic M, Garcia-Pascual A, De Vente J, Persson K. Activity and expression of nitric oxide synthase in the hypertrophied rat bladder and the effect of nitric oxide on bladder smooth muscle growth. J Urol. 2002; 168:2689–2694.
Article20. Matsumoto S, Hanai T, Uemura H. Chronic treatment with a PDE5 inhibitor increases contractile force of normal bladder in rats. Int Urol Nephrol. 2010; 42:53–56.
Article21. Dombkowski RA, Doellman MM, Head SK, Olson KR. Hydrogen sulfide mediates hypoxia-induced relaxation of trout urinary bladder smooth muscle. J Exp Biol. 2006; 209:3234–3240.
Article22. Patacchini R, Santicioli P, Giuliani S, Maggi CA. Pharmacological investigation of hydrogen sulfide (H2S) contractile activity in rat detrusor muscle. Eur J Pharmacol. 2005; 509:171–177.
Article23. Patacchini R, Santicioli P, Giuliani S, Maggi CA. Hydrogen sulfide (H2S) stimulates capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol. 2004; 142:31–34.
Article24. Mustafa S, Ismael HN. Reactivity of diabetic urinary bladder to the cholinesterase inhibitor neostigmine. Urology. 2014; 84:1549.e1–1549.e5.
Article25. Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol. 2006; 176:380–386.
Article26. Longhurst PA, Belis JA. Abnormalities of rat bladder contractility in streptozotocin-induced diabetes mellitus. J Pharmacol Exp Ther. 1986; 238:773–777.27. Je HD, Park SY, Barber AL, Sohn UD. The inhibitory effect of rosiglitazone on agonist-induced or spontaneous regulation of contractility. Arch Pharm Res. 2007; 30:461–468.
Article28. Waring JV, Wendt IR. Effects of streptozotocin-induced diabetes mellitus on intracellular calcium and contraction of longitudinal smooth muscle from rat urinary bladder. J Urol. 2000; 163:323–330.
Article29. Belis JA, Curley RM, Wagner CH, Murty VN, Winter SJ, Rohner TJ Jr. Neurogenic function of the diabetic rat bladder: alteration by calcium channel effectors. Pharmacology. 1991; 43:273–281.
Article30. Poladia DP, Bauer JA. Early cell-specific changes in nitric oxide synthases, reactive nitrogen species formation, and ubiquitinylation during diabetes-related bladder remodeling. Diabetes Metab Res Rev. 2003; 19:313–319.
Article31. Adela R, Nethi SK, Bagul PK, Barui AK, Mattapally S, Kuncha M, Patra CR, Reddy PN, Banerjee SK. Hyperglycaemia enhances nitric oxide production in diabetes: a study from South Indian patients. PLoS One. 2015; 10:e0125270.
Article32. Golbidi S, Laher I. Bladder dysfunction in diabetes mellitus. Front Pharmacol. 2010; 1:136.
Article33. Huang L, Chen D, Li S. Streptozotocin diabetes attenuates the effects of nondepolarizing neuromuscular relaxants on rat muscles. Korean J Physiol Pharmacol. 2014; 18:461–467.
Article34. Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006; 147:Suppl 2. S88–S119.35. Kudlacz EM, Gerald MC, Wallace LJ. Effects of diabetes and diuresis on contraction and relaxation mechanisms in rat urinary bladder. Diabetes. 1989; 38:278–284.
Article36. Yu W, Zacharia LC, Jackson EK, Apodaca G. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol. 2006; 291:C254–C265.
Article37. Martinson EA, Johnson RA, Wells JN. Potent adenosine receptor antagonists that are selective for the A1 receptor subtype. Mol Pharmacol. 1987; 31:247–252.38. Lohse MJ, Klotz KN, Lindenborn-Fotinos J, Reddington M, Schwabe U, Olsson RA. 8-Cyclopentyl-,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987; 336:204–210.39. Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003; 15:813–827.
Article40. Bucheimer RE, Linden J. Purinergic regulation of epithelial transport. J Physiol. 2004; 555:311–321.
Article41. Inoue R, Brading AF. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990; 100:619–625.
Article42. Zhu X, Zhai K, Mi Y, Ji G. Expression and function of phosphodiesterases (PDEs) in the rat urinary bladder. BMC Urol. 2017; 17:54.
Article43. Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003; 93:280–291.
Article44. Tong YC, Chin WT, Cheng JT. Alterations in urinary bladder M2-muscarinic receptor protein and mRNA in 2-week streptozotocininduced diabetic rats. Neurosci Lett. 1999; 277:173–176.
Article45. Sand C, Michel MC. Bradykinin contracts rat urinary bladder largely independently of phospholipase C. J Pharmacol Exp Ther. 2014; 348:25–31.
Article46. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003; 83:1325–1358.
Article47. Levy J, Gavin JR 3rd, Sowers JR. Diabetes mellitus: a disease of abnormal cellular calcium metabolism. Am J Med. 1994; 96:260–273.
Article48. Kim SJ, Park JH, Song DK, Park KS, Lee JE, Kim ES, Cho KB, Jang BK, Chung WJ, Hwang JS, Kwon JG, Kim TW. Alterations of colonic contractility in long-term diabetic rat model. J Neurogastroenterol Motil. 2011; 17:372–380.
Article49. Iltis I, Kober F, Desrois M, Dalmasso C, Lan C, Portha B, Cozzone PJ, Bernard M. Defective myocardial blood flow and altered function of the left ventricle in type 2 diabetic rats: a noninvasive in vivo study using perfusion and cine magnetic resonance imaging. Invest Radiol. 2005; 40:19–26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Inhibitory Mechanism on Acetylcholine-Induced Contraction of Bladder Smooth Muscle in the Streptozotocin-Induced Diabetic Rat
- Expression of Caveolin-1 in Rat Urinary Bladder with Cyclophosphamide-Induced Cystitis
- The Effects of Botulinum Toxin A on the Detrusor Muscle in Rats
- Changes in the endothelin-1-induced contraction of aorta in streptozotocin-induced diabetic rats.
- Partial Outlet Obstruction Induced Alterations of Rat Urinary Bladder Muscle