Yonsei Med J.
2017 Nov;58(6):1195-1203. 10.3349/ymj.2017.58.6.1195.
Effects of Autologous Platelet-Rich Plasma on Regeneration of Damaged Endometrium in Female Rats
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea. cyberpelvis@gmail.com
- 2Department of Biomedical Sciences, Korea University College of Medicine, Seoul, Korea.
- 3Division of Developmental Biology and Physiology, School of Biosciences and Chemistry, Institute for Basic Sciences, Sungshin University, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, CHA Bundang Medical Center, CHA University College of Medicine, Seongnam, Korea. callen1013@gmail.com
- KMID: 2418902
- DOI: http://doi.org/10.3349/ymj.2017.58.6.1195
Abstract
- PURPOSE
To investigate whether autologous platelet-rich plasma (PRP) treatment can improve regeneration of the endometrium in an experimental model of ethanol-induced damage.
MATERIALS AND METHODS
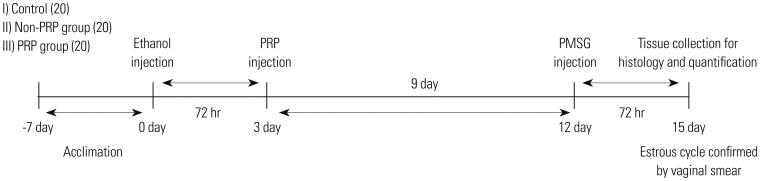
Sixty female Sprague-Dawley rats were randomly assigned into three groups: control group, ethanol group, and PRP-treated group (administration of 0.25 mL of PRP into both uterine cavities 72 hours after ethanol injection). After 15 days of endometrial damage, all the animals were sacrificed during the estrous cycle, and samples were taken from the mid-uterine horn. Functional and structural recovery of the endometrium was analyzed by hematoxylin-eosin (H&E) and Masson trichrome (MT) staining, real-time polymerase chain reaction (PCR) assay, and immuno-histochemical (IHC) analyses.
RESULTS
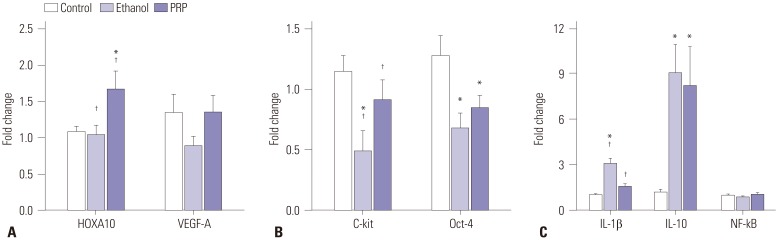
H&E and MT staining confirmed significantly decreased fibrosis and increased cellular proliferation in the PRP-treated group, compared to the ethanol group. The endometrial areas in the ethanol and PRP-treated groups were 212.83±15.84 µm² and 262.34±12.33 µm² (p=0.065). Significantly stronger IHC expression of cytokeratin, homeobox A10 (HOXA10), vascular endothelial growth factor (VEGF), and Ki-67 was found in the PRP-treated group, compared to the ethanol group. In real-time PCR analyses, interleukin-1β mRNA was down-regulated, while c-Kit mRNA was up-regulated, in the PRP-treated group, compared to the ethanol group.
CONCLUSION
Intrauterine administration of autologous PRP stimulated and accelerated regeneration of the endometrium and also decreased fibrosis in a murine model of damaged endometrium.
MeSH Terms
-
Animals
Cell Proliferation
Endometrium/*metabolism
Female
Interleukin-1beta
Platelet-Rich Plasma/*metabolism
Random Allocation
Rats
Rats, Sprague-Dawley
Real-Time Polymerase Chain Reaction
Regeneration/*physiology
Vascular Endothelial Growth Factor A/*metabolism
Interleukin-1beta
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Gargett CE, Healy DL. Generating receptive endometrium in Asherman's syndrome. J Hum Reprod Sci. 2011; 4:49–52. PMID: 21772741.2. Senturk LM, Erel CT. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008; 20:221–228. PMID: 18460935.
Article3. Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome--one century later. Fertil Steril. 2008; 89:759–779. PMID: 18406834.
Article4. Chen MJ, Yang JH, Peng FH, Chen SU, Ho HN, Yang YS. Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet. 2006; 23:337–342. PMID: 16983519.
Article5. Khairy M, Banerjee K, El-Toukhy T, Coomarasamy A, Khalaf Y. Aspirin in women undergoing in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2007; 88:822–831. PMID: 17509593.
Article6. Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002; 78:1073–1076. PMID: 12413996.
Article7. Takasaki A, Tamura H, Miwa I, Taketani T, Shimamura K, Sugino N. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil Steril. 2010; 93:1851–1858. PMID: 19200982.
Article8. Cervelló I, Gil-Sanchis C, Santamaría X, Cabanillas S, Díaz A, Faus A, et al. Human CD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil Steril. 2015; 104:1552–1560.e1-3. PMID: 26384164.
Article9. Alawadhi F, Du H, Cakmak H, Taylor HS. Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One. 2014; 9:e96662. PMID: 24819371.
Article10. Jing Z, Qiong Z, Yonggang W, Yanping L. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat. Fertil Steril. 2014; 101:587–594. PMID: 24355044.
Article11. Zhao J, Zhang Q, Wang Y, Li Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod Sci. 2015; 22:181–188. PMID: 24947483.
Article12. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007; 25:2082–2086. PMID: 17464086.
Article13. Figueira PG, Abrão MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2011; 1221:10–17. PMID: 21401624.
Article14. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001; 10:225–228. PMID: 11813662.
Article15. El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007; 78:661–669. PMID: 17397313.
Article16. Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004; 70:1738–1750. PMID: 14766732.17. Chegini N, Rossi MJ, Masterson BJ. Platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and EGF and PDGF beta-receptors in human endometrial tissue: localization and in vitro action. Endocrinology. 1992; 130:2373–2385. PMID: 1312455.
Article18. Tang XM, Zhao Y, Rossi MJ, Abu-Rustum RS, Ksander GA, Chegini N. Expression of transforming growth factor-beta (TGF beta) isoforms and TGF beta type II receptor messenger ribonucleic acid and protein, and the effect of TGF beta s on endometrial stromal cell growth and protein degradation in vitro. Endocrinology. 1994; 135:450–459. PMID: 8013384.
Article19. Everts PA, Knape JT, Weibrich G, Schönberger JP, Hoffmann J, Overdevest EP, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006; 38:174–187. PMID: 16921694.20. Jing Z, Hong G, Yanping L. Development of an animal model for thin endometrium using 95% ethanol. J Fert In Vitro. 2012; 2:4.
Article21. Li Y, Pan P, Chen X, Li L, Li Y, Yang D. Granulocyte colony-stimulating factor administration for infertile women with thin endometrium in frozen embryo transfer program. Reprod Sci. 2014; 21:381–385. PMID: 23885097.
Article22. Kon H, Tohei A, Hokao R, Shinoda M. Estrous cycle stage-independent treatment of PMSG and hCG can induce superovulation in adult Wistar-Imamichi rats. Exp Anim. 2005; 54:185–187. PMID: 15897629.
Article23. DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012; 28:998–1009. PMID: 22738751.
Article24. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012; 18:1028–1040. PMID: 22772564.
Article25. Terada S, Ota S, Kobayashi M, Kobayashi T, Mifune Y, Takayama K, et al. Use of an antifibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury. J Bone Joint Surg Am. 2013; 95:980–988. PMID: 23780535.
Article26. Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014; 9:e96801. PMID: 24802416.
Article27. Tuominen VJ, Ruotoistenmäki S, Viitanen A, Jumppanen M, Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 2010; 12:R56. PMID: 20663194.
Article28. Surrey ES, Halme J. Effect of platelet-derived growth factor on endometrial stromal cell proliferation in vitro: a model for endometriosis? Fertil Steril. 1991; 56:672–679. PMID: 1915941.
Article29. Munson L, Upadhyaya NB, Van Meter S. Platelet-derived growth factor promotes endometrial epithelial cell proliferation. Am J Obstet Gynecol. 1995; 173:1820–1825. PMID: 8610769.
Article30. Bagot CN, Kliman HJ, Taylor HS. Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev Dyn. 2001; 222:538–544. PMID: 11747087.
Article31. Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005; 65:550–563. PMID: 15664381.
Article32. Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013; 9:721–730. PMID: 24080861.
Article33. Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. Int Immunol. 1999; 11:1781–1790. PMID: 10545482.34. Szabo G, Mandrekar P, Catalano D. Inhibition of superantigen-induced T cell proliferation and monocyte IL-1 beta, TNF-alpha, and IL-6 production by acute ethanol treatment. J Leukoc Biol. 1995; 58:342–350. PMID: 7665990.35. Cervelló I, Mas A, Gil-Sanchis C, Peris L, Faus A, Saunders PT, et al. Reconstruction of endometrium from human endometrial side population cell lines. PLoS One. 2011; 6:e21221. PMID: 21712999.
Article36. Cho NH, Park YK, Kim YT, Yang H, Kim SK. Lifetime expression of stem cell markers in the uterine endometrium. Fertil Steril. 2004; 81:403–407. PMID: 14967381.
Article37. Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016; 22:137–163. PMID: 26552890.
Article38. Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015; 8:1286–1290. PMID: 25785127.39. Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Ajori L. Effects of autologous platelet-rich plasma on implantation and pregnancy in repeated implantation failure: a pilot study. Int J Reprod Biomed (Yazd). 2016; 14:625–628. PMID: 27921085.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Platelet-rich Plasma on Burn Wounds according to Time of Application: An Experimental Study on Rats
- Current clinical applications of platelet-rich plasma in various gynecological disorders: An appraisal of theory and practice
- Intralesional Injection of Autologous Platelet-Rich Plasma as an Effective Regeneration Therapy: A Case Report of Chronic Wagner Grade 2 Diabetic Foot Ulcer
- Platelet-rich plasma treatment in patients with refractory thin endometrium and recurrent implantation failure: A comprehensive review
- Effects of Autologous Platelet-Rich Plasma on Postoperative Blood Loss and Transfusion Requirements in Cardiac Surgery