Obstet Gynecol Sci.
2017 Nov;60(6):506-519. 10.5468/ogs.2017.60.6.506.
What is fetal programming?: a lifetime health is under the control of in utero health
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Ewha Womans University College of Medicine, Seoul, Korea. kkyj@ewha.ac.kr
- KMID: 2418351
- DOI: http://doi.org/10.5468/ogs.2017.60.6.506
Abstract
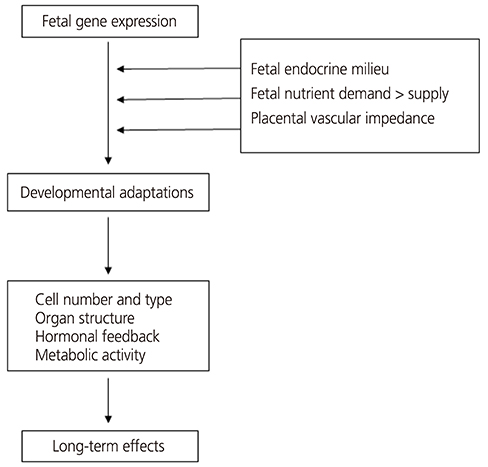
- The "Barker hypothesis" postulates that a number of organ structures and associated functions undergo programming during embryonic and fetal life, which determines the set point of physiological and metabolic responses that carry into adulthood. Hence, any stimulus or insult at a critical period of embryonic and fetal development can result in developmental adaptations that produce permanent structural, physiological and metabolic changes, thereby predisposing an individual to cardiovascular, metabolic and endocrine disease in adult life. This article will provide evidence linking these diseases to fetal undernutrition and an overview of previous studies in this area as well as current advances in understanding the mechanism and the role of the placenta in fetal programming.
MeSH Terms
Figure
Cited by 3 articles
-
Long-term effects of pro-opiomelanocortin methylation induced in food-restricted dams on metabolic phenotypes in male rat offspring
Sangmi Lee, Eun Jin Kwon, Young-Ah You, Ji Eun Du, Inho Jo, Young Ju Kim
Obstet Gynecol Sci. 2020;63(3):239-250. doi: 10.5468/ogs.2020.63.3.239.Fetal programming: could intrauterin life affect health status in adulthood?
Hande Nur Onur Öztürk, Perim Fatma Türker
Obstet Gynecol Sci. 2021;64(6):473-483. doi: 10.5468/ogs.21154.Effects of Maternal Diet during Pregnancy or Lactation on the Development or Prevention of Allergic Diseases in Offspring
Ja Kyoung Kim
J Korean Soc Matern Child Health. 2022;26(3):121-131. doi: 10.21896/jksmch.2022.26.3.121.
Reference
-
1. Barker DJ. In utero programming of chronic disease. Clin Sci (Lond). 1998; 95:115–128.2. Widdowson EM, McCance RA. A review: new thoughts on growth. Pediatr Res. 1975; 9:154–156.3. Lucas A. Programming by early nutrition in man. In : Bock GR, Whelan J, editors. The childhood environment and adult disease. Chichester: John Wiley & Sons;1991. p. 38–55.4. Lucas A. Role of nutritional programming in determining adult morbidity. Arch Dis Child. 1994; 71:288–290.5. Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995; 311:171–174.6. Barker DJ. Mothers, babies, and health in later life. 2nd ed. Edinburgh: Churchill Livingstone;1998.7. Fowden AL. Endocrine regulation of fetal growth. Reprod Fertil Dev. 1995; 7:351–363.8. Harding JE, Johnston BM. Nutrition and fetal growth. Reprod Fertil Dev. 1995; 7:539–547.9. Campbell AG, Dawes GS, Fishman AP, Hyman AI. Regional redistribution of blood flow in the mature fetal lamb. Circ Res. 1967; 21:229–235.10. Rudolph AM. The fetal circulation and its response to stress. J Dev Physiol. 1984; 6:11–19.11. Fowden AL. The role of insulin in prenatal growth. J Dev Physiol. 1989; 12:173–182.12. Oliver MH, Harding JE, Breier BH, Evans PC, Gluckman PD. Glucose but not a mixed amino acid infusion regulates plasma insulin-like growth factor-I concentrations in fetal sheep. Pediatr Res. 1993; 34:62–65.13. Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004; 127:515–526.14. Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993; 307:1519–1524.15. Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997; 13:807–813.16. Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993; 36:62–67.17. Leon DA, Lithell HO, Vâgerö D, Koupilová I, Mohsen R, Berglund L, et al. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915–29. BMJ. 1998; 317:241–245.18. Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ. 1996; 312:406–410.19. Barker DJ, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002; 31:1235–1239.20. Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996; 14:935–941.21. Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001; 185:93–98.22. Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991; 303:1019–1022.23. Phillips DI, Hirst S, Clark PM, Hales CN, Osmond C. Fetal growth and insulin secretion in adult life. Diabetologia. 1994; 37:592–596.24. Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992; 35:595–601.25. Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001; 60:5–20.26. Hales CN, Ozanne SE. The dangerous road of catch-up growth. J Physiol. 2003; 547:5–10.27. Godfrey KM. The role of the placenta in fetal programming-a review. Placenta. 2002; 23:Suppl A. S20–S27.28. Cunningham S, Cameron IT. Consequences of fetal growth restriction during childhood and adult life. Curr Obstet Gynaecol. 2003; 13:212–217.29. Reik W, Constância M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, et al. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol. 2003; 547:35–44.30. Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf). 1997; 46:161–166.31. Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11beta-hydroxysteroid dehydrogenase, and fetal programming. Kidney Int. 2000; 57:1412–1417.32. Vaughan OR, Forhead AJ, Fowden AL. Glucocorticoids and placental programming. In : Burton GJ, Barker DJ, Moffett A, Thornburg KL, editors. The placenta and human developmental programming. Cambridge: Cambridge University Press;2011. p. 175–187.33. Lesage J, Blondeau B, Grino M, Bréant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001; 142:1692–1702.34. Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993; 341:355–357.35. Sloboda DM, Newnham JP, Moss TJ, Challis JR. The fetal hypothalamic-pituitary-adrenal axis: relevance to developmental origins of health and disease. In : Gluckman PD, Hanson MA, editors. Developmental origins of health and disease. New York (NY): Cambridge University Press;2006. p. 191–205.36. Jellyman JK, Valenzuela OA, Fowden AL. Horse species symposium: glucocorticoid programming of hypothalamic-pituitary-adrenal axis and metabolic function: animal studies from mouse to horse. J Anim Sci. 2015; 93:3245–3260.37. Brown RW, Diaz R, Robson AC, Kotelevtsev YV, Mullins JJ, Kaufman MH, et al. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996; 137:794–797.38. Mackenzie HS, Lawler EV, Brenner BM. Congenital oligonephropathy: the fetal flaw in essential hypertension? Kidney Int Suppl. 1996; 55:S30–S34.39. Mañalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000; 58:770–773.40. Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. BMJ. 1993; 307:1524–1527.41. Barker DJ, Meade TW, Fall CH, Lee A, Osmond C, Phipps K, et al. Relation of fetal and infant growth to plasma fibrinogen and factor VII concentrations in adult life. BMJ. 1992; 304:148–152.42. Leeson CP, Whincup PH, Cook DG, Donald AE, Papacosta O, Lucas A, et al. Flow-mediated dilation in 9- to 11-year-old children: the influence of intrauterine and childhood factors. Circulation. 1997; 96:2233–2238.43. Veille JC, Hanson R, Sivakoff M, Hoen H, Ben-Ami M. Fetal cardiac size in normal, intrauterine growth retarded, and diabetic pregnancies. Am J Perinatol. 1993; 10:275–279.44. Murotsuki J, Challis JR, Han VK, Fraher LJ, Gagnon R. Chronic fetal placental embolization and hypoxemia cause hypertension and myocardial hypertrophy in fetal sheep. Am J Physiol. 1997; 272:R201–R207.45. van Assche FA, Aerts L. The fetal endocrine pancreas. Contrib Gynecol Obstet. 1979; 5:44–57.46. Fall CH, Stein CE, Kumaran K, Cox V, Osmond C, Barker DJ, et al. Size at birth, maternal weight, and type 2 diabetes in South India. Diabet Med. 1998; 15:220–227.47. Fowden AL, Coan PM, Angiolini E, Burton GJ, Constancia M. Imprinted genes and the epigenetic regulation of placental phenotype. Prog Biophys Mol Biol. 2011; 106:281–288.48. Byrne CD. Programming other hormones that affect insulin. Br Med Bull. 2001; 60:153–171.49. Breier BH, Vickers MH, Ikenasio BA, Chan KY, Wong WP. Fetal programming of appetite and obesity. Mol Cell Endocrinol. 2001; 185:73–79.50. Lee S, Lee KA, Choi GY, Desai M, Lee SH, Pang MG, et al. Feed restriction during pregnancy/lactation induces programmed changes in lipid, adiponectin and leptin levels with gender differences in rat offspring. J Matern Fetal Neonatal Med. 2013; 26:908–914.51. Forhead AJ, Fowden AL. The hungry fetus? Role of leptin as a nutritional signal before birth. J Physiol. 2009; 587:1145–1152.52. Cooper C, Javaid MK, Taylor P, Walker-Bone K, Dennison E, Arden N. The fetal origins of osteoporotic fracture. Calcif Tissue Int. 2002; 70:391–394.53. Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996; 175:1534–1542.54. Jansson T, Ekstrand Y, Björn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002; 51:2214–2219.55. Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006; 572:25–30.56. Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997; 277:1669–1672.57. Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996; 19:157–167.58. Lee JH, Yoo JY, You YA, Kwon WS, Lee SM, Pang MG, et al. Proteomic analysis of fetal programming-related obesity markers. Proteomics. 2015; 15:2669–2677.59. You YA, Lee JH, Kwon EJ, Yoo JY, Kwon WS, Pang MG, et al. Proteomic analysis of one-carbon metabolism-related marker in liver of rat offspring. Mol Cell Proteomics. 2015; 14:2901–2909.60. Lee S, You YA, Kwon EJ, Jung SC, Jo I, Kim YJ. Maternal food restriction during pregnancy and lactation adversely affect hepatic growth and lipid metabolism in three-week-old rat offspring. Int J Mol Sci. 2016; 17:E2115.61. Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005; 135:1382–1386.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- What is fetal programming?: A lifetime health is under the control of in-utero health
- Fetal programming: could intrauterin life affect health status in adulthood?
- Fetal Programming and Adult Disease
- Clinical Analysis of Intrauterine Fetal Death in Dongsan Medical Center for Recent Five Years
- The Maternal Obesity: Adverse Outcomes of Pregnancy and the Offspring