Korean J Orthod.
2018 Jul;48(4):253-261. 10.4041/kjod.2018.48.4.253.
Effect of caspases and RANKL induced by heavy force in orthodontic root resorption
- Affiliations
-
- 1Department of Orthodontics, Nihon University School of Dentistry at Matsudo, Chiba, Japan. yamaguchi.masaru@nihon-u.ac.jp
- 2Department of Oral Pathology, Nihon University School of Dentistry at Matsudo, Chiba, Japan.
- KMID: 2417967
- DOI: http://doi.org/10.4041/kjod.2018.48.4.253
Abstract
OBJECTIVE
Orthodontic root resorption (ORR) due to orthodontic tooth movement is a difficult treatment-related adverse event. Caspases are important effector molecules for apoptosis. At present, little is known about the mechanisms underlying ORR and apoptosis in the cementum. The aim of the present in vivo study was to investigate the expression of tartrate-resistant acid phosphatase (TRAP), caspase 3, caspase 8, and receptor activator of nuclear factor kappa-B ligand (RANKL) in the cementum in response to a heavy or an optimum orthodontic force.
METHODS
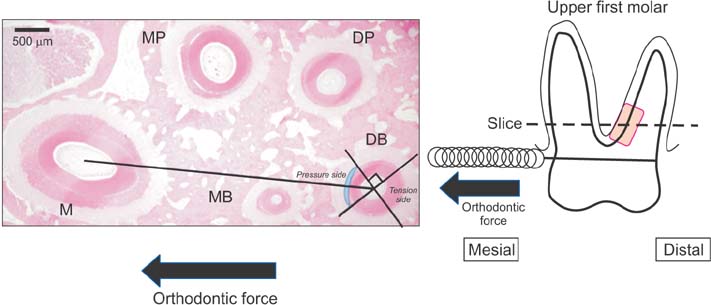
The maxillary molars of male Wistar rats were subjected to an orthodontic force of 10 g or 50 g using a closed coil spring. The rats were sacrificed each experimental period on days 1, 3, 5, and 7 after orthodontic force application. And the rats were subjected to histopathological and immunohistochemical analyses.
RESULTS
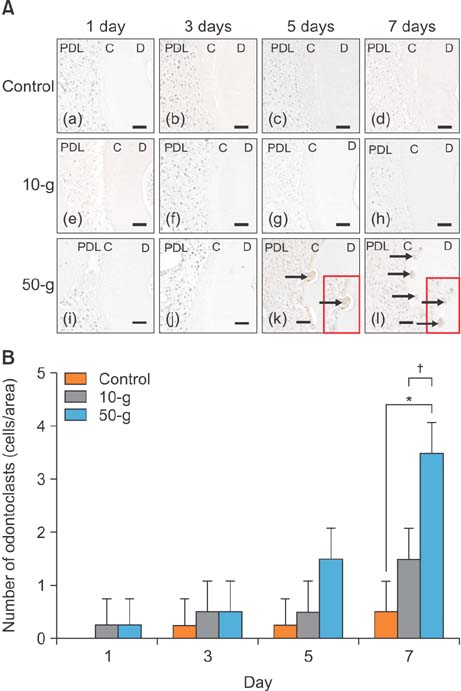
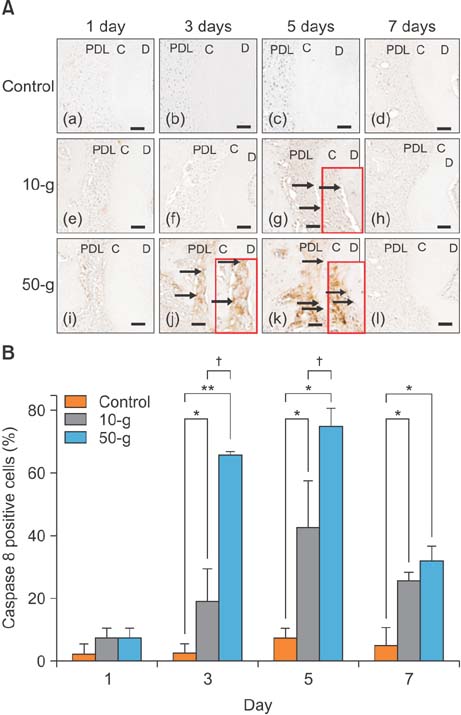
On day 7 for the 50-g group, hematoxylin and eosin staining revealed numerous root resorption lacunae with odontoclasts on the root, while immunohistochemistry showed increased TRAP- and RANKL-positive cells. Caspase 3- and caspase 8-positive cells were increased on the cementum surfaces in the 50-g group on days 3 and 5. Moreover, the number of caspase 3- and caspase 8-positive cells and RANKL-positive cells was significantly higher in the 50-g group than in the 10-g group.
CONCLUSIONS
In our rat model, ORR occurred after apoptosis was induced in the cementum by a heavy orthodontic force. These findings suggest that apoptosis of cementoblasts is involved in ORR.
MeSH Terms
Figure
Reference
-
1. Kaley J, Phillips C. Factors related to root resorption in edgewise practice. Angle Orthod. 1991; 61:125–132.2. Linge BO, Linge L. Apical root resorption in upper anterior teeth. Eur J Orthod. 1983; 5:173–183.
Article3. Owman-Moll P, Kurol J, Lundgren D. The effects of a four-fold increased orthodontic force magnitude on tooth movement and root resorptions. An intra-individual study in adolescents. Eur J Orthod. 1996; 18:287–294.
Article4. Owman-Moll P, Kurol J, Lundgren D. Effects of a doubled orthodontic force magnitude on tooth movement and root resorptions. An inter-individual study in adolescents. Eur J Orthod. 1996; 18:141–150.
Article5. Chan E, Darendeliler MA. Physical properties of root cementum: Part 5. Volumetric analysis of root resorption craters after application of light and heavy orthodontic forces. Am J Orthod Dentofacial Orthop. 2005; 127:186–195.
Article6. Hikida T, Yamaguchi M, Shimizu M, Kikuta J, Yoshino T, Kasai K. Comparisons of orthodontic root resorption under heavy and jiggling reciprocating forces during experimental tooth movement in a rat model. Korean J Orthod. 2016; 46:228–241.
Article7. Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001; 11:372–377.
Article8. Hamaya M, Mizoguchi I, Sakakura Y, Yajima T, Abiko Y. Cell death of osteocytes occurs in rat alveolar bone during experimental tooth movement. Calcif Tissue Int. 2002; 70:117–126.
Article9. Rana MW, Pothisiri V, Killiany DM, Xu XM. Detection of apoptosis during orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2001; 119:516–521.
Article10. Wu CC, Lin JP, Yang JS, Chou ST, Chen SC, Lin YT, et al. Capsaicin induced cell cycle arrest and apoptosis in human esophagus epidermoid carcinoma CE 81T/VGH cells through the elevation of intracellular reactive oxygen species and Ca2+ productions and caspase-3 activation. Mutat Res. 2006; 601:71–82.
Article11. Diercke K, Zingler S, Kohl A, Lux CJ, Erber R. Gene expression profile of compressed primary human cementoblasts before and after IL-1β stimulation. Clin Oral Investig. 2014; 18:1925–1939.
Article12. Nakano Y, Yamaguchi M, Fujita S, Asano M, Saito K, Kasai K. Expressions of RANKL/RANK and M-CSF/c-fms in root resorption lacunae in rat molar by heavy orthodontic force. Eur J Orthod. 2011; 33:335–343.
Article13. Hatai T, Yokozeki M, Funato N, Baba Y, Moriyama K, Ichijo H, et al. Apoptosis of periodontal ligament cells induced by mechanical stress during tooth movement. Oral Dis. 2001; 7:287–290.
Article14. Asano M, Yamaguchi M, Nakajima R, Fujita S, Utsunomiya T, Yamamoto H, et al. IL-8 and MCP-1 induced by excessive orthodontic force mediates odontoclastogenesis in periodontal tissues. Oral Dis. 2011; 17:489–498.
Article15. Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, et al. A unified model for apical caspase activation. Mol Cell. 2003; 11:529–541.
Article16. Mabuchi R, Matsuzaka K, Shimono M. Cell proliferation and cell death in periodontal ligaments during orthodontic tooth movement. J Periodontal Res. 2002; 37:118–124.
Article17. Zheng L, Wang W, Ni J, Mao X, Song D, Liu T, et al. Role of autophagy in tumor necrosis factor-α-induced apoptosis of osteoblast cells. J Investig Med. 2017; 65:1014–1020.
Article18. Mitsuhashi M, Yamaguchi M, Kojima T, Nakajima R, Kasai K. Effects of HSP70 on the compression force-induced TNF-α and RANKL expression in human periodontal ligament cells. Inflamm Res. 2011; 60:187–194.
Article19. Matsumoto Y, Sringkarnboriboon S, Ono T. Proinflammatory mediators related to orthodontically induced periapical root resorption in rat mandibular molars. Eur J Orthod. 2017; 39:686–691.
Article20. Szymczyk KH, Freeman TA, Adams CS, Srinivas V, Steinbeck MJ. Active caspase-3 is required for osteoclast differentiation. J Cell Physiol. 2006; 209:836–844.
Article21. Kagayama M, Sasano Y, Mizoguchi I, Takahashi I. Confocal microscopy of cementocytes and their lacunae and canaliculi in rat molars. Anat Embryol (Berl). 1997; 195:491–496.
Article22. Ayasaka N, Kondo T, Goto T, Kido MA, Nagata E, Tanaka T. Differences in the transport systems between cementocytes and osteocytes in rats using microperoxidase as a tracer. Arch Oral Biol. 1992; 37:363–369.
Article23. Sasano Y, Maruya Y, Sato H, Zhu JX, Takahashi I, Mizoguchi I, et al. Distinctive expression of extracellular matrix molecules at mRNA and protein levels during formation of cellular and acellular cementum in the rat. Histochem J. 2001; 33:91–99.24. Matsuzawa H, Toriya N, Nakao Y, Konno-Nagasaka M, Arakawa T, Okayama M, et al. Cementocyte cell death occurs in rat cellular cementum during orthodontic tooth movement. Angle Orthod. 2017; 87:416–422.
Article25. Brudvik P, Rygh P. Root resorption beneath the main hyalinized zone. Eur J Orthod. 1994; 16:249–263.
Article26. Kurol J, Owman-Moll P. Hyalinization and root resorption during early orthodontic tooth movement in adolescents. Angle Orthod. 1998; 68:161–165.27. Kvam E. Scanning electron microscopy of tissue changes on the pressure surface of human premolars following tooth movement. Scand J Dent Res. 1972; 80:357–368.
Article28. Rygh P. Ultrastructural cellular reactions in pressure zones of rat molar periodontium incident to orthodontic tooth movement. Acta Odontol Scand. 1972; 30:575–593.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effet of types of orthodontic force on the root resorption and repair in rat molar
- Comparisons of orthodontic root resorption under heavy and jiggling reciprocating forces during experimental tooth movement in a rat model
- Mechanisms of Osteoclastogenesis in Orthodontic Tooth Movement and Orthodontically Induced Tooth Root Resorption
- Root resorption and bone resorption by jiggling force in cat premolars
- Effect of orthodontic force on the amount of tooth movement and root resorption in rat