Korean J Pain.
2018 Jul;31(3):174-182. 10.3344/kjp.2018.31.3.174.
Orexin-A inhibits capsaicin-induced changes in cyclooxygenase-2 and brain-derived neurotrophic factor expression in trigeminal nucleus caudalis of rats
- Affiliations
-
- 1Department of Biology, Faculty of Sciences, Shahid Bahonar University of Kerman, Kerman, Iran.
- 2Endodontology Research Center, Kerman University of Medical Sciences, Kerman, Iran. Maryam.raoof@gmail.com
- 3Laboratory of Molecular Neuroscience, Neuroscience Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran.
- 4Pathology and Stem Cell Research Center, Department of Pathology, Afzalipour Kerman University of Medical Science, Kerman, Iran.
- KMID: 2417955
- DOI: http://doi.org/10.3344/kjp.2018.31.3.174
Abstract
- BACKGROUND
The trigeminal nucleus caudalis (Vc) is a primary central site for trigeminal transmitting. Noxious stimulation of the trigeminal nociceptors alters the central synaptic releases and neural expression of some inflammatory and trophic agents. Orexin-A and the orexin 1 receptor (OX1R) are expressed in pain pathways including trigeminal pain transmission. However, the the mechanism(s) underling orexin-A effects on trigeminal pain modulation have not been fully clarified.
METHODS
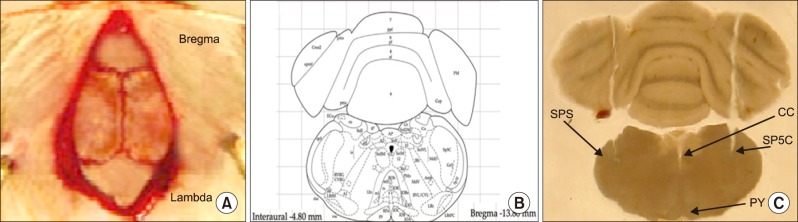
Trigeminal pain was induced by subcutaneous injection of capsaicin in the upper lip in rats. The effect of trigeminal pain on cyclooxygenase-2 (COX-2) and brain-derived neurotrophic factor (BDNF) expression in the Vc of animals was determined by immunofluorescence. Subsequently, OX1R agonist (orexin-A) and antagonist (SB-334867-A) was administrated in the Vc to investigate the possible roles of the Vc OX1R on changes in COX-2 and BDNF levels following pain induction.
RESULTS
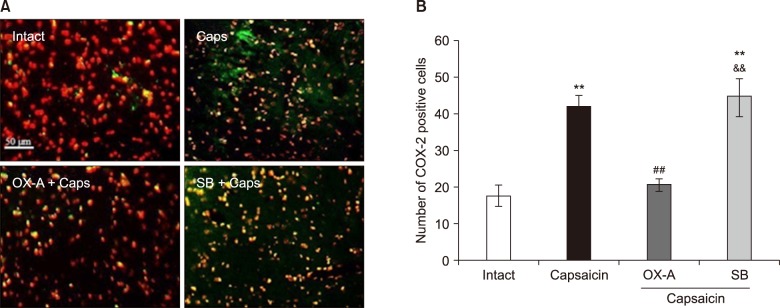
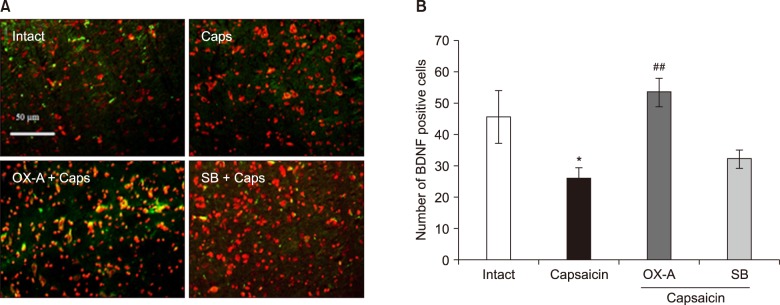
The data indicated an increase in COX-2 and decrease in BDNF immuno-reactivity in the Vc of capsaicin, and capsaicin- pretreated with SB-334867-A (80 nM), groups of rat. However, the effect of capsaicin on COX-2 and BDNF expressions was reversed by a Vc microinjection of orexin-A (100 pM).
CONCLUSIONS
Overall, the present data reveals that orexin-A can attenuate capsaicin-induced trigeminal pain through the modulation of pain effects on COX-2 and BDNF expressions in the Vc of rats.
Keyword
MeSH Terms
-
Animals
Brain-Derived Neurotrophic Factor*
Capsaicin
Cyclooxygenase 2*
Facial Pain
Fluorescent Antibody Technique
Injections, Subcutaneous
Lip
Microinjections
Nociceptors
Orexin Receptor Antagonists
Orexins*
Pain Measurement
Pain Perception
Rats*
Trigeminal Caudal Nucleus
Trigeminal Neuralgia
Trigeminal Nuclei*
Brain-Derived Neurotrophic Factor
Capsaicin
Cyclooxygenase 2
Orexin Receptor Antagonists
Orexins
Figure
Cited by 1 articles
-
The ability of orexin-A to modify pain-induced cyclooxygenase-2 and brain-derived neurotrophic factor expression is associated with its ability to inhibit capsaicin-induced pulpal nociception in rats
Fatemeh Shahsavari, Mehdi Abbasnejad, Saeed Esmaeili-Mahani, Maryam Raoof
Korean J Pain. 2022;35(3):261-270. doi: 10.3344/kjp.2022.35.3.261.
Reference
-
1. Sessle BJ. Neural mechanisms and pathways in craniofacial pain. Can J Neurol Sci. 1999; 26(Suppl 3):S7–S11.
Article2. Boggero IA, Rojas-Ramirez MV, de Leeuw R, Carlson CR. Satisfaction with life in orofacial pain disorders: associations and theoretical implications. J Oral Facial Pain Headache. 2016; 30:99–106. PMID: 27128473.
Article3. Gao Y, Duan YZ. Increased COX2 in the trigeminal nucleus caudalis is involved in orofacial pain induced by experimental tooth movement. Anat Rec (Hoboken). 2010; 293:485–491. PMID: 20091889.
Article4. Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, et al. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci. 2004; 19:3375–3381. PMID: 15217394.
Article5. Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008; 89:312–323. PMID: 17942328.
Article6. Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, et al. BDNF as a pain modulator. Prog Neurobiol. 2008; 85:297–317. PMID: 18514997.
Article7. Zhu ZW, Friess H, Wang L, Zimmermann A, Büchler MW. Brain-derived neurotrophic factor (BDNF) is upregulated and associated with pain in chronic pancreatitis. Dig Dis Sci. 2001; 46:1633–1639. PMID: 11508661.8. Price TJ, Louria MD, Candelario-Soto D, Dussor GO, Jeske NA, Patwardhan AM, et al. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005; 6:4. PMID: 15667652.9. Shu XQ, Llinas A, Mendell LM. Effects of trkB and trkC neurotrophin receptor agonists on thermal nociception: a behavioral and electrophysiological study. Pain. 1999; 80:463–470. PMID: 10342408.
Article10. Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008; 13:717–728. PMID: 17700577.
Article11. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998; 92:573–585. PMID: 9491897.
Article12. Holland PR, Akerman S, Goadsby PJ. Modulation of nociceptive dural input to the trigeminal nucleus caudalis via activation of the orexin 1 receptor in the rat. Eur J Neurosci. 2006; 24:2825–2833. PMID: 17156207.
Article13. Akbari E, Motamedi F, Davoodi FG, Noorbakhshnia M, Ghanbarian E. Orexin-1 receptor mediates long-term potentiation in the dentate gyrus area of freely moving rats. Behav Brain Res. 2011; 216:375–380. PMID: 20728473.
Article14. Holland PR, Akerman S, Goadsby PJ. Orexin 1 receptor activation attenuates neurogenic dural vasodilation in an animal model of trigeminovascular nociception. J Pharmacol Exp Ther. 2005; 315:1380–1385. PMID: 16160082.
Article15. Kooshki R, Abbasnejad M, Esmaeili-Mahani S, Raoof M. The role of trigeminal nucleus caudalis orexin 1 receptors in orofacial pain transmission and in orofacial pain-induced learning and memory impairment in rats. Physiol Behav. 2016; 157:20–27. PMID: 26821188.
Article16. Paxinos G, Franklin KB. The rat brain in stereotaxic coordinates [electronic resource]. 4th ed. San Diego (CA): Academic Press;1998.17. Raoof M, Shakoori A, Kooshki R, Abbasnejad M, Amanpour S. The effects of regular exercise on capsaicin-induced pulpal pain and pain-induced changes in passive avoidance learning and memory in rats. Korean J Pain. 2017; 30:258–264. PMID: 29123620.
Article18. Ahn DK, Choi HS, Yeo SP, Woo YW, Lee MK, Yang GY, et al. Blockade of central cyclooxygenase (COX) pathways enhances the cannabinoid-induced antinociceptive effects on inflammatory temporomandibular joint (TMJ) nociception. Pain. 2007; 132:23–32. PMID: 17321048.
Article19. Zhou Y, Long H, Ye N, Liao L, Yang X, Jian F, et al. The effect of capsaicin on expression patterns of CGRP in trigeminal ganglion and trigeminal nucleus caudalis following experimental tooth movement in rats. J Appl Oral Sci. 2016; 24:597–606. PMID: 28076465.
Article20. Quartu M, Serra MP, Boi M, Poddighe L, Picci C, Demontis R, et al. TRPV1 receptor in the human trigeminal ganglion and spinal nucleus: immunohistochemical localization and comparison with the neuropeptides CGRP and SP. J Anat. 2016; 229:755–767. PMID: 27456865.
Article21. Kistner K, Siklosi N, Babes A, Khalil M, Selescu T, Zimmermann K, et al. Systemic desensitization through TRPA1 channels by capsazepine and mustard oil - a novel strategy against inflammation and pain. Sci Rep. 2016; 6:28621. PMID: 27356469.
Article22. Neeb L, Hellen P, Boehnke C, Hoffmann J, Schuh-Hofer S, Dirnagl U, et al. IL-1β stimulates COX-2 dependent PGE2 synthesis and CGRP release in rat trigeminal ganglia cells. PLoS One. 2011; 6:e17360. PMID: 21394197.23. Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain. 2009; 5:43. PMID: 19660121.
Article24. Hood DD, Curry R, Eisenach JC. Intravenous remifentanil produces withdrawal hyperalgesia in volunteers with capsaicin-induced hyperalgesia. Anesth Analg. 2003; 97:810–815. PMID: 12933407.
Article25. Iwaoka E, Wang S, Matsuyoshi N, Kogure Y, Aoki S, Yamamoto S, et al. Evodiamine suppresses capsaicin-induced thermal hyperalgesia through activation and subsequent desensitization of the transient receptor potential V1 channels. J Nat Med. 2016; 70:1–7. PMID: 26188960.
Article26. Hay C, de Belleroche J. Carrageenan-induced hyperalgesia is associated with increased cyclo-oxygenase-2 expression in spinal cord. Neuroreport. 1997; 8:1249–1251. PMID: 9175123.
Article27. Butterick TA, Nixon JP, Billington CJ, Kotz CM. Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model. Neurosci Lett. 2012; 524:30–34. PMID: 22796468.
Article28. Cheng JK, Chou RC, Hwang LL, Chiou LC. Antiallodynic effects of intrathecal orexins in a rat model of postoperative pain. J Pharmacol Exp Ther. 2003; 307:1065–1071. PMID: 14551290.
Article29. Cady RJ, Denson JE, Sullivan LQ, Durham PL. Dual orexin receptor antagonist 12 inhibits expression of proteins in neurons and glia implicated in peripheral and central sensitization. Neuroscience. 2014; 269:79–92. PMID: 24685439.
Article30. Xiong X, White RE, Xu L, Yang L, Sun X, Zou B, et al. Mitigation of murine focal cerebral ischemia by the hypocretin/orexin system is associated with reduced inflammation. Stroke. 2013; 44:764–770. PMID: 23349191.
Article31. Zhan S, Cai GQ, Zheng A, Wang Y, Jia J, Fang H, et al. Tumor necrosis factor-alpha regulates the hypocretin system via mRNA degradation and ubiquitination. Biochim Biophys Acta. 2011; 1812:565–571. PMID: 21094253.
Article32. Leszek J, Barreto GE, Gąsiorowski K, Koutsouraki E, Ávila-Rodrigues M, Aliev G. Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: role of brain innate immune system. CNS Neurol Disord Drug Targets. 2016; 15:329–336. PMID: 26831258.
Article33. Raoof M, Esmaeili-Mahani S, Nourzadeh M, Raoof R, Abbasnejad M, Amirkhosravi L, et al. Noxious stimulation of the rat tooth pulp may impair learning and memory through the induction of hippocampal apoptosis. J Oral Facial Pain Headache. 2015; 29:390–397. PMID: 26485387.
Article34. Pasban-Aliabadi H, Esmaeili-Mahani S, Abbasnejad M. Orexin-A protects human neuroblastoma SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity: involvement of PKC and PI3K signaling pathways. Rejuvenation Res. 2017; 20:125–133. PMID: 27814668.
Article35. Kitamura E, Hamada J, Kanazawa N, Yonekura J, Masuda R, Sakai F, et al. The effect of orexin-A on the pathological mechanism in the rat focal cerebral ischemia. Neurosci Res. 2010; 68:154–157. PMID: 20600373.
Article36. Ichikawa H, Yabuuchi T, Jin HW, Terayama R, Yamaai T, Deguchi T, et al. Brain-derived neurotrophic factor-immunoreactive primary sensory neurons in the rat trigeminal ganglion and trigeminal sensory nuclei. Brain Res. 2006; 1081:113–118. PMID: 16510129.
Article37. Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem. 2006; 99:1338–1350. PMID: 17064360.
Article38. Behnia A, Zhang L, Charles M, Gold MS. Changes in TrkB-like immunoreactivity in rat trigeminal ganglion after tooth injury. J Endod. 2003; 29:135–140. PMID: 12597715.
Article39. Tarsa L, Bałkowiec-Iskra E, Kratochvil FJ 3rd, Jenkins VK, McLean A, Brown AL, et al. Tooth pulp inflammation increases brain-derived neurotrophic factor expression in rodent trigeminal ganglion neurons. Neuroscience. 2010; 167:1205–1215. PMID: 20223282.
Article40. Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007; 133:9–17. PMID: 17379413.
Article41. Aubdool AA, Kodji X, Abdul-Kader N, Heads R, Fernandes ES, Bevan S, et al. TRPA1 activation leads to neurogenic vasodilatation: involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br J Pharmacol. 2016; 173:2419–2433. PMID: 27189253.
Article42. Kapczinski F, Frey BN, Andreazza AC, Kauer-Sant'Anna M, Cunha AB, Post RM. Increased oxidative stress as a mechanism for decreased BDNF levels in acute manic episodes. Rev Bras Psiquiatr. 2008; 30:243–245. PMID: 18833425.
Article43. Harada S, Yamazaki Y, Tokuyama S. Orexin-A suppresses postischemic glucose intolerance and neuronal damage through hypothalamic brain-derived neurotrophic factor. J Pharmacol Exp Ther. 2013; 344:276–285. PMID: 23117790.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The ability of orexin-A to modify pain-induced cyclooxygenase-2 and brain-derived neurotrophic factor expression is associated with its ability to inhibit capsaicin-induced pulpal nociception in rats
- Fos Protein Expression in Trigeminal Nociceptive Central Pathway of the Rat Brain by Cisternal Capsaicin Injection
- The Antinociceptive Effect of Sigma-1 Receptor Antagonist, BD1047, in a Capsaicin Induced Headache Model in Rats
- Expression and Distribution of BDNF (Brain Derived Neurotrophic Factor) in the Rat Hypothalamus
- Distribution of Brain-Derived Neurotrophic Factor-Immunoreactive Neurons in the Rat Brain after Colchicine Treatment