Cancer Res Treat.
2018 Jul;50(3):964-974. 10.4143/crt.2017.346.
The Prognostic Impact of the Number of Metastatic Lymph Nodes and a New Prognostic Scoring System for Recurrence in Early-Stage Cervical Cancer with High Risk Factors: A Multicenter Cohort Study (KROG 15-04)
- Affiliations
-
- 1Department of Radiation Oncology, Chungnam National University College of Medicine, Daejeon, Korea.
- 2Department of Radiation Oncology, Seoul National University Bundang Hospital, Seongnam, Korea. 978sarang@hanmail.net
- 3Department of Radiation Oncology, Asan Medical center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Radiation Oncology, Ajou University School of Medicine, Suwon, Korea.
- 6Department of Radiation Oncology, Ewha Womans University Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea.
- 7Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 8Department of Radiation Oncology, Korea University Ansan Hospital, Ansan, Korea.
- 9Department of Radiation Oncology, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
- 10Department of Radiation Oncology, Chung-Ang University Hospital, Seoul, Korea.
- 11Department of Radiation Oncology, CHA Bundang Medicial Center, CHA University School of Medicine, Seongnam, Korea.
- 12Department of Radiation Oncology, Gyeongsang National University Hospital, Jinju, Korea.
- 13Department of Radiation Oncology, Gachon University Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea.
- 14Department of Radiation Oncology, Wonju Severance Christian Hospital, Wonju, Korea.
- KMID: 2417885
- DOI: http://doi.org/10.4143/crt.2017.346
Abstract
- PURPOSE
We aimed to assess prognostic value of metastatic pelvic lymph node (mPLN) in early-stage cervical cancer treated with radical surgery followed by postoperative chemoradiotherapy. Also, we sought to define a high-risk group using prognosticators for recurrence.
MATERIALS AND METHODS
A multicenter retrospective study was conducted using the data from 13 Korean institutions from 2000 to 2010. A total of 249 IB-IIA patients with high-risk factors were included. We evaluated distant metastasis-free survival (DMFS) and disease-free survival (DFS) in relation to clinicopathologic factors including pNstage, number of mPLN, lymph node (LN)ratio (number of positive LN/number of harvested LN), and log odds of mPLNs (log(number of positive LN+0.5/number of negative LN+0.5)).
RESULTS
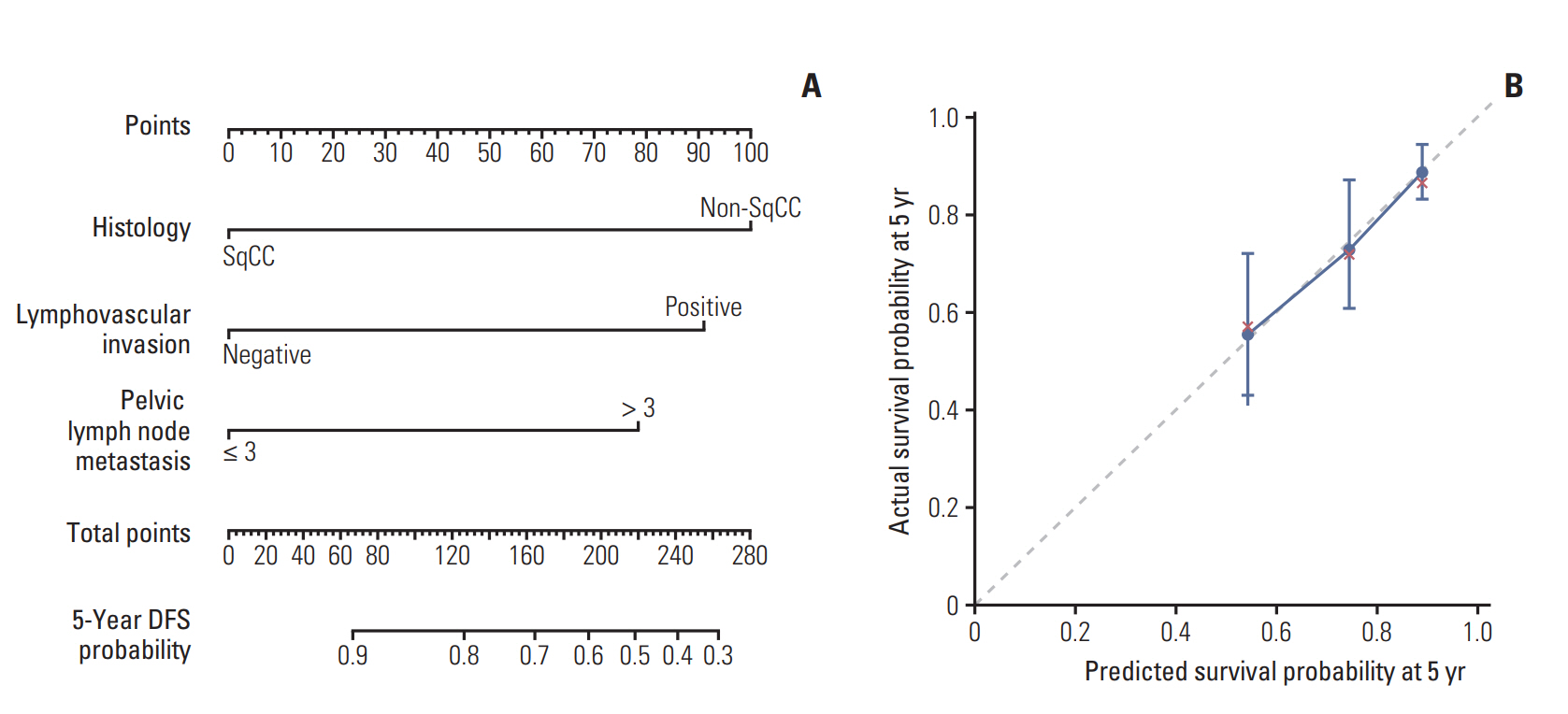
In univariate analysis, histology (squamous cell carcinoma [SqCC] vs. others), lymphovascular invasion (LVI), number of mPLNs (≤ 3 vs. > 3), LN ratio (≤ 17% vs. > 17%), and log odds of mPLNs (≤ -0.58 vs. > -0.58) were significant prognosticators for DMFS and DFS. Resection margin involvement only affected DFS. No significant survival difference was observed between pN0 patients and patients with 1-3 mPLNs. Multivariate analysis revealed that mPLN > 3, LVI, and non-SqCC were unfavorable index for both DMFS (p < 0.001, p=0.020, and p=0.031, respectively) and DFS (p < 0.001, p=0.017, and p=0.001, respectively). A scoring system using these three factors predicts risk of recurrence with relatively high concordance index (DMFS, 0.69; DFS, 0.71).
CONCLUSION
mPLN > 3 in early-stage cervical cancer affects DMFS and DFS. A scoring system using mPLNs > 3, LVI, and non-SqCC could stratify risk groups of recurrence in surgically resected early-stage cervix cancer with high-risk factors.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The prognostic value of lymph node ratio in stage IIIC cervical cancer patients triaged to primary treatment by radical hysterectomy with systematic pelvic and para-aortic lymphadenectomy
Koray Aslan, Mehmet Mutlu Meydanli, Murat Oz, Yusuf Aytac Tohma, Ali Haberal, Ali Ayhan
J Gynecol Oncol. 2020;31(1):. doi: 10.3802/jgo.2020.31.e1.
Reference
-
References
1. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017; 49:292–305.
Article2. Moon EK, Oh CM, Won YJ, Lee JK, Jung KW, Cho H, et al. Trends and age-period-cohort effects on the incidence and mortality rate of cervical cancer in Korea. Cancer Res Treat. 2017; 49:526–33.
Article3. Nowakowski A, Cybulski M, Buda I, Janosz I, Olszak-Wasik K, Bodzek P, et al. Cervical cancer histology, staging and survival before and after implementation of organised cervical screening programme in Poland. PLoS One. 2016; 11:e0155849.
Article4. Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997; 350:535–40.
Article5. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000; 18:1606–13.
Article6. Kidd EA, Siegel BA, Dehdashti F, Rader JS, Mutch DG, Powell MA, et al. Lymph node staging by positron emission tomography in cervical cancer: relationship to prognosis. J Clin Oncol. 2010; 28:2108–13.
Article7. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER cancer statistics review 1975-2013 [Internet]. Bethesda, MD: National Cancer Institute;2016. [cited 2016 Dec 26]. Available from: https://seer.cancer.gov/csr/1975_2013/.8. Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990; 38:352–7.
Article9. Stehman FB, Bundy BN, DiSaia PJ, Keys HM, Larson JE, Fowler WC. Carcinoma of the cervix treated with radiation therapy. I. A multi-variate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991; 67:2776–85.
Article10. Tsai CS, Lai CH, Wang CC, Chang JT, Chang TC, Tseng CJ, et al. The prognostic factors for patients with early cervical cancer treated by radical hysterectomy and postoperative radiotherapy. Gynecol Oncol. 1999; 75:328–33.
Article11. Lee HJ, Han S, Kim YS, Nam JH, Kim HJ, Kim JW, et al. Individualized prediction of overall survival after postoperative radiation therapy in patients with early-stage cervical cancer: a Korean Radiation Oncology Group study (KROG 13-03). Int J Radiat Oncol Biol Phys. 2013; 87:659–64.
Article12. Kwon J, Eom KY, Kim IA, Kim JS, Kim YB, No JH, et al. Prognostic value of log odds of positive lymph nodes after radical surgery followed by adjuvant treatment in high-risk cervical cancer. Cancer Res Treat. 2016; 48:632–40.
Article13. Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009; 105:107–8.
Article14. Compton CC, Byrd DR, Garcia-Aguilar J, Kurtzman SH, Olawaiye A, Washington MK. AJCC cancer staging atlas: a companion to the seventh editions of the AJCC cancer staging manual and handbook. New York: Springer;2012.15. Monk BJ, Wang J, Im S, Stock RJ, Peters WA 3rd, Liu PY, et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol Oncol. 2005; 96:721–8.
Article16. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999; 73:177–83.
Article17. Fleming ND, Frumovitz M, Schmeler KM, dos Reis R, Munsell MF, Eifel PJ, et al. Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol Oncol. 2015; 136:48–53.
Article18. Li C, Liu W, Cheng Y. Prognostic significance of metastatic lymph node ratio in squamous cell carcinoma of the cervix. Onco Targets Ther. 2016; 9:3791–7.19. Polterauer S, Hefler L, Seebacher V, Rahhal J, Tempfer C, Horvat R, et al. The impact of lymph node density on survival of cervical cancer patients. Br J Cancer. 2010; 103:613–6.
Article20. Chen Y, Zhang L, Tian J, Fu X, Ren X, Hao Q. Significance of the absolute number and ratio of metastatic lymph nodes in predicting postoperative survival for the International Federation of Gynecology and Obstetrics stage IA2 to IIA cervical cancer. Int J Gynecol Cancer. 2013; 23:157–63.
Article21. Zhou J, Sun JY, Chen SY, Li FY, Lin HX, Wu SG, et al. Prognostic value of lymph node ratio in patients with small-cell carcinoma of the cervix based on data from a large national registry. Onco Targets Ther. 2015; 9:67–73.22. Aurello P, Petrucciani N, Nigri GR, La Torre M, Magistri P, Tierno S, et al. Log odds of positive lymph nodes (LODDS): what are their role in the prognostic assessment of gastric adenocarcinoma? J Gastrointest Surg. 2014; 18:1254–60.
Article23. Morice P, Piovesan P, Rey A, Atallah D, Haie-Meder C, Pautier P, et al. Prognostic value of lymphovascular space invasion determined with hematoxylin-eosin staining in early stage cervical carcinoma: results of a multivariate analysis. Ann Oncol. 2003; 14:1511–7.
Article24. Chandacham A, Charoenkwan K, Siriaunkgul S, Srisomboon J, Suprasert P, Phongnarisorn C, et al. Extent of lymphovascular space invasion and risk of pelvic lymph node metastases in stage IB1 cervical cancer. J Med Assoc Thai. 2005; 88 Suppl 2:S31–6.25. Hosaka M, Watari H, Mitamura T, Konno Y, Odagiri T, Kato T, et al. Survival and prognosticators of node-positive cervical cancer patients treated with radical hysterectomy and systematic lymphadenectomy. Int J Clin Oncol. 2011; 16:33–8.
Article26. Kim HJ, Rhee WJ, Choi SH, Nam EJ, Kim SW, Kim S, et al. Clinical outcomes of adjuvant radiation therapy and prognostic factors in early stage uterine cervical cancer. Radiat Oncol J. 2015; 33:126–33.
Article27. Galic V, Herzog TJ, Lewin SN, Neugut AI, Burke WM, Lu YS, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012; 125:287–91.
Article28. Intaraphet S, Kasatpibal N, Siriaunkgul S, Sogaard M, Patumanond J, Khunamornpong S, et al. Prognostic impact of histology in patients with cervical squamous cell carcinoma, adenocarcinoma and small cell neuroendocrine carcinoma. Asian Pac J Cancer Prev. 2013; 14:5355–60.
Article29. Winer I, Alvarado-Cabrero I, Hassan O, Ahmed QF, Alosh B, Bandyopadhyay S, et al. The prognostic significance of histologic type in early stage cervical cancer: a multi-institutional study. Gynecol Oncol. 2015; 137:474–8.30. Mabuchi S, Okazawa M, Matsuo K, Kawano M, Suzuki O, Miyatake T, et al. Impact of histological subtype on survival of patients with surgically-treated stage IA2-IIB cervical cancer: adenocarcinoma versus squamous cell carcinoma. Gynecol Oncol. 2012; 127:114–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Factors For Survival in Patients with Lymph Node Metastasis Identified at the Time of Surgery for Cervical Carcinoma
- Proposal of the Nodal Stage Based on the Number of Metastatic Lymph Nodes in Patients with Gastric Cancer
- Prognostic value of the lymph node metastasis in patients with ampulla of Vater cancer after surgical resection
- A Distribution Weighted Prognostic Scoring Model for Node Status in Advanced Rectal Cancer
- Simplified prognostic factor scoring system in patients with lymph node-negative stage IB-IIA cervical cancer