Cancer Res Treat.
2018 Jul;50(3):801-812. 10.4143/crt.2017.210.
Feasibility of Charcoal Tattooing of Cytology-Proven Metastatic Axillary Lymph Node at Diagnosis and Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Breast Cancer Patients
- Affiliations
-
- 1Division of Breast Surgery, Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 2Frontier Research Institute of Convergence Sports Science, Yonsei University, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Division of Medical Oncology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Radiology and Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. mines@yuhs.ac
- KMID: 2417869
- DOI: http://doi.org/10.4143/crt.2017.210
Abstract
- PURPOSE
Sentinel lymph node biopsy (SLNB) can be performed when node-positive disease is converted to node-negative status after neoadjuvant chemotherapy (NCT). Tattooing nodes might improve accuracy but supportive data are limited. This study aimed to investigate the feasibility of charcoal tattooing metastatic axillary lymph node (ALN) at presentation followed by SLNB after NCT in breast cancers.
MATERIALS AND METHODS
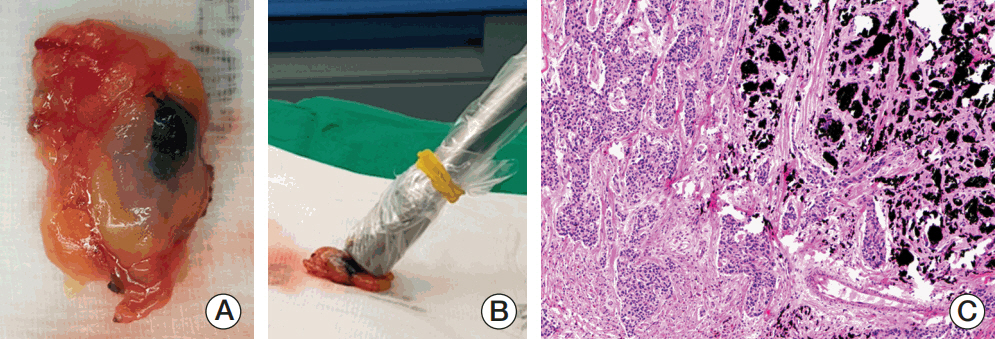
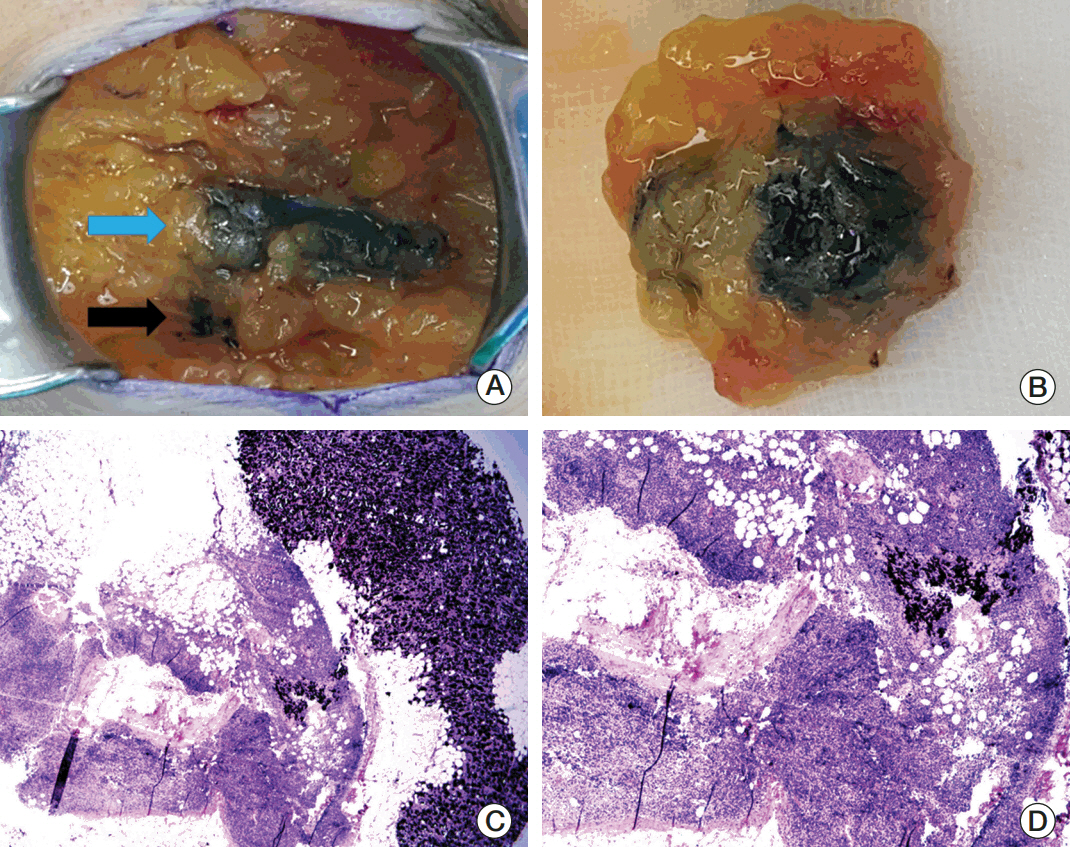
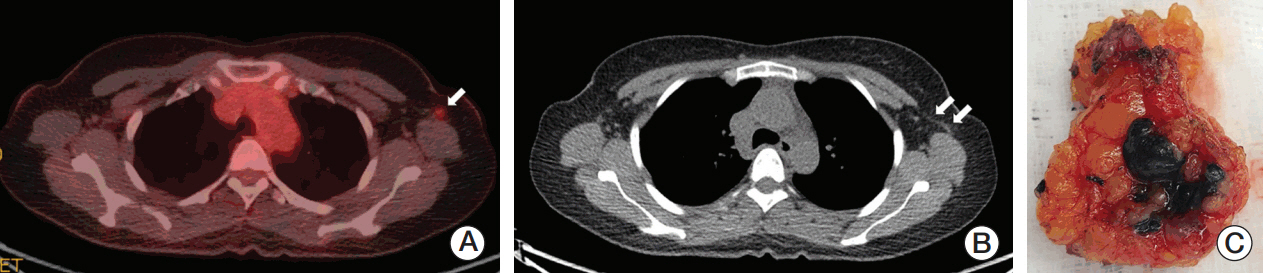
Twenty patientswith cytology-proven node metastases prospectively underwent charcoal tattooing at diagnosis. SLNB using dual tracers and axillary surgery after NCT were then performed. The detection rate of tattooed node and diagnostic performance of SLNB were analyzed.
RESULTS
All patients underwent charcoal tattooingwithout significant morbidity. Sentinel and tattooed nodes could be detected during surgery after NCT. Nodal pathologic complete response was achieved in 10 patients. Overall sensitivity, false-negative rate (FNR), negative predictive value, and accuracy of hot/blue SLNB were 80.0%, 20.0%, 83.3%, and 90.0%, respectively. Retrieving more nodes and favorable nodal response were associated with improved performance. The best accuracy was observed when excised tattooed node was calculated together (FNR, 0.0%). Cold/non-blue tattooed nodes of five patients were removed during non-sentinel axillary surgery but clinicopathological parameters did not differ compared to patients with hot/blue tattooed node detected during SLNB, suggesting the importance of the tattooing procedure itself to improve performance.
CONCLUSION
Charcoal tattooing of cytology-confirmed metastatic ALN at presentation is technically feasible and does not limit SLNB after NCT. The tattooing procedure without additional preoperative localization is advantageous for improving the diagnostic performance of SLNB in this setting.
MeSH Terms
Figure
Cited by 1 articles
-
Targeted axillary biopsy and sentinel lymph node biopsy for axillary restaging after neoadjuvant chemotherapy
Gunay Gurleyik, Sibel Aydin Aksu, Fügen Aker, Kubra Kaytaz Tekyol, Eda Tanrikulu, Emin Gurleyik
Ann Surg Treat Res. 2021;100(6):305-312. doi: 10.4174/astr.2021.100.6.305.
Reference
-
References
1. Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017; 35:561–4.
Article2. Bromham N, Schmidt-Hansen M, Astin M, Hasler E, Reed MW. Axillary treatment for operable primary breast cancer. Cochrane Database Syst Rev. 2017; 1:CD004561.
Article3. Park S, Park JM, Cho JH, Park HS, Kim SI, Park BW. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with cytologically proven node-positive breast cancer at diagnosis. Ann Surg Oncol. 2013; 20:2858–65.
Article4. Pilewskie M, Morrow M. Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol. 2017; 3:549–55.5. Jatoi I, Benson JR, Toi M. De-escalation of axillary surgery in early breast cancer. Lancet Oncol. 2016; 17:e430–41.
Article6. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013; 14:609–18.
Article7. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–61.8. Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015; 33:258–64.
Article9. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Invasive breast cancer version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016; 14:324–54.10. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016; 34:1072–8.
Article11. Choy N, Lipson J, Porter C, Ozawa M, Kieryn A, Pal S, et al. Initial results with preoperative tattooing of biopsied axillary lymph nodes and correlation to sentinel lymph nodes in breast cancer patients. Ann Surg Oncol. 2015; 22:377–82.
Article12. Shin K, Caudle AS, Kuerer HM, Santiago L, Candelaria RP, Dogan B, et al. Radiologic mapping for targeted axillary dissection: needle biopsy to excision. AJR Am J Roentgenol. 2016; 207:1372–9.
Article13. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.14. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–95.
Article15. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.
Article16. Weaver DL. Pathology evaluation of sentinel lymph nodes in breast cancer: protocol recommendations and rationale. Mod Pathol. 2010; 23 Suppl 2:S26–32.
Article17. Glechner A, Wockel A, Gartlehner G, Thaler K, Strobelberger M, Griebler U, et al. Sentinel lymph node dissection only versus complete axillary lymph node dissection in early invasive breast cancer: a systematic review and meta-analysis. Eur J Cancer. 2013; 49:812–25.
Article18. Mastrangelo S, McMasters K, Ajkay N. Surgical management of the axilla in breast cancer. Am Surg. 2016; 82:475–86.19. Rubio IT. Sentinel lymph node biopsy after neoadjuvant treatment in breast cancer: work in progress. Eur J Surg Oncol. 2016; 42:326–32.
Article20. van Nijnatten TJ, Schipper RJ, Lobbes MB, Nelemans PJ, BeetsTan RG, Smidt ML. The diagnostic performance of sentinel lymph node biopsy in pathologically confirmed node positive breast cancer patients after neoadjuvant systemic therapy: a systematic review and meta-analysis. Eur J Surg Oncol. 2015; 41:1278–87.
Article21. El Hage Chehade H, Headon H, El Tokhy O, Heeney J, Kasem A, Mokbel K. Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3,398 patients. Am J Surg. 2016; 212:969–81.
Article22. King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015; 12:335–43.
Article23. Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol. 2016; 23:3467–74.
Article24. Boughey JC, Ballman KV, Hunt KK, McCall LM, Mittendorf EA, Ahrendt GM, et al. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the American College of Surgeons Oncology Group Z1071 Trial (Alliance). J Clin Oncol. 2015; 33:3386–93.
Article25. Enokido K, Watanabe C, Nakamura S, Ogiya A, Osako T, Akiyama F, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with an initial diagnosis of cytology-proven lymph node-positive breast cancer. Clin Breast Cancer. 2016; 16:299–304.
Article26. Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005; 23:7703–20.
Article27. Amersi F, Giuliano AE. Management of the axilla. Hematol Oncol Clin North Am. 2013; 27:687–702.
Article28. Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results From ACOSOG Z1071 (Alliance). Ann Surg. 2016; 263:802–7.29. Kawase K, Gayed IW, Hunt KK, Kuerer HM, Akins J, Yi M, et al. Use of lymphoscintigraphy defines lymphatic drainage patterns before sentinel lymph node biopsy for breast cancer. J Am Coll Surg. 2006; 203:64–72.
Article30. Ando J, Kitamura T, Kuroki Y, Igarashi S. Preoperative diagnosis of the axillary arch with multidetector row computed tomography and the axillary arch in association with anatomical problems of sentinel lymph node biopsy. Breast Cancer. 2010; 17:3–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update
- The Number of Removed Lymph Nodes for an Acceptable False Negative Rate in Sentinel Lymph Node Biopsy for Breast Cancer
- Prospective Evaluation of the Feasibility of Sentinel Lymph Node Biopsy in Breast Cancer Patients with Negative Axillary Conversion after Neoadjuvant Chemotherapy
- Sentinel Lymph Node Biopsy in Patients with Clinically Negative Lymph Node After Neoadjuvant Chemotherapy
- Use of Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Patients with Axillary Node-Positive Breast Cancer in Diagnosis