J Pathol Transl Med.
2018 Jul;52(4):238-242. 10.4132/jptm.2017.11.21.
Primary Cutaneous Mucinous Carcinoma with Extramammary Paget’s Disease: Eccrine or Apocrine?
- Affiliations
-
- 1Department of Pathology, Kosin University Gospel Hospital, Busan, Korea. 10highpowerfield@gmail.com
- KMID: 2417808
- DOI: http://doi.org/10.4132/jptm.2017.11.21
Abstract
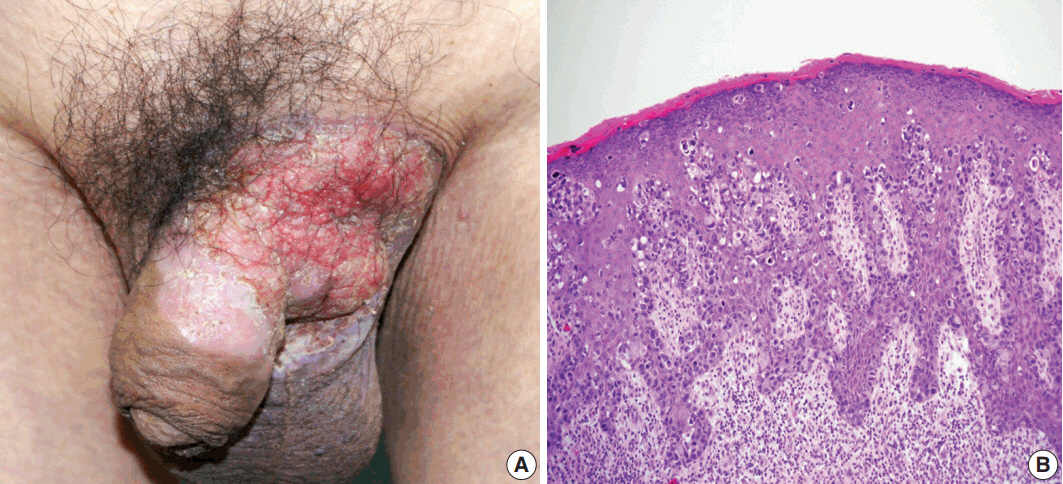
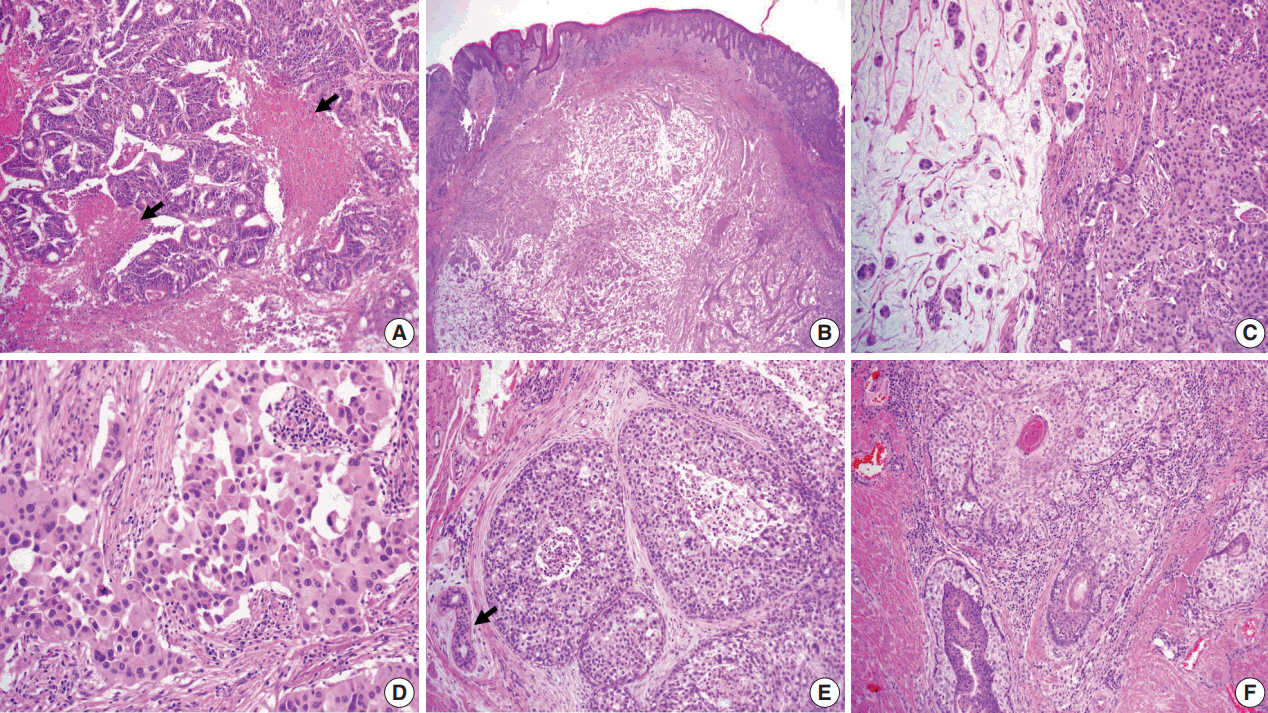
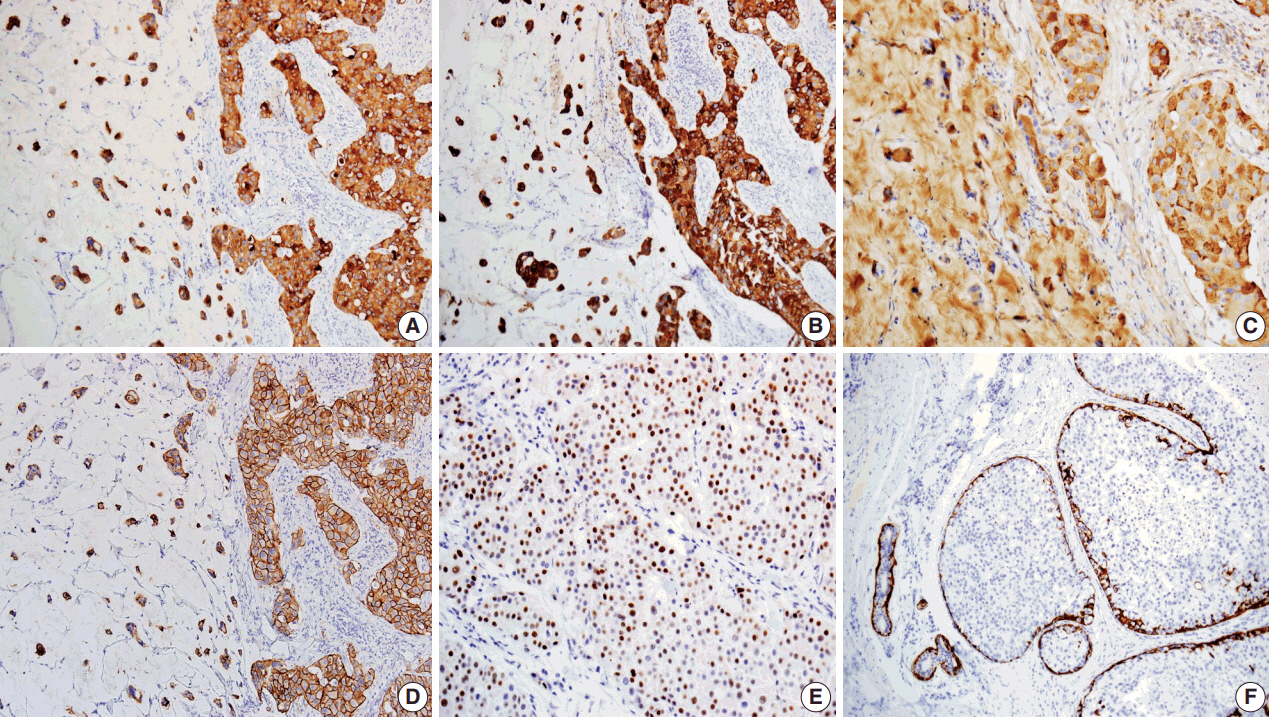
- Primary cutaneous mucinous carcinoma (PCMC) is an uncommon tumor of the sweat gland origin. The occurrence of PCMC is mostly in middle-aged and older patients, with a slight male predominance. Most cases of PCMC arise on the head, with a preference for eyelids. The histogenesis of PCMC, whether eccrine or apocrine, remains controversial. We report a rare case of PCMC with secondary extramammary Paget's disease in the groin of a 75-year-old man, which favored an apocrine origin. Furthermore, based on a review of the literature, we provide several histologic clues that can be used to differentiate PCMC from metastatic mucinous carcinoma.
Keyword
MeSH Terms
Figure
Reference
-
1. Kazakov DV, Suster S, LeBoit PE, et al. Mucinous carcinoma of the skin, primary, and secondary: a clinicopathologic study of 63 cases with emphasis on the morphologic spectrum of primary cutaneous forms: homologies with mucinous lesions in the breast. Am J Surg Pathol. 2005; 29:764–82.2. Qureshi HS, Salama ME, Chitale D, et al. Primary cutaneous mucinous carcinoma: presence of myoepithelial cells as a clue to the cutaneous origin. Am J Dermatopathol. 2004; 26:353–8.
Article3. Headington JT. Primary mucinous carcinoma of skin: histochemistry and electron microscopy. Cancer. 1977; 39:1055–63.4. Wright JD, Font RL. Mucinous sweat gland adenocarcinoma of eyelid: a clinicopathologic study of 21 cases with histochemical and electron microscopic observations. Cancer. 1979; 44:1757–68.5. Pilgrim JP, Kloss SG, Wolfish PS, Heng MC. Primary mucinous carcinoma of the skin with metastases to the lymph nodes. Am J Dermatopathol. 1985; 7:461–9.
Article6. Martinez S, Young S. Primary mucinous carcinoma of the skin. Internet J Oncol. 2004; 2:1–7.7. Requena L, Kiryu H, Ackerman AB. Neoplasms with apocrine differentiation. Philadelphia: Lippincott Williams & Wilkins;1998. p. 907–47.8. Saigal RK, Khanna SD, Chander J. Apocrine gland carcinoma in axilla. Indian J Dermatol Venereol. 1971; 37:177–80.9. Aso K, Sato N. A case of primary mucinous carcinoma of the skin and trichofolliculoma developed in the same tissue. Jpn J Clin Dermatol. 1989; 43:117–20.10. Wako M, Nishimaki K, Kawamura N, et al. Mucinous carcinoma of the skin with apocrine-type differentiation: immunohistochemical studies. Am J Dermatopathol. 2003; 25:66–70.11. Lennox B, Pearse AG, Richards HG. Mucin-secreting tumours of the skin with special reference to the so-called mixed-salivary tumour of the skin and its relation to hidradenoma. J Pathol Bacteriol. 1952; 64:865–80.
Article12. Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008; 32:682–90.13. Helwig EB, Graham JH. Anogenital (extramammary) Paget’s disease: a clinicopathological study. Cancer. 1963; 16:387–403.14. Pascual JC, Perez-Ramos M, Devesa JP, Kutzner H, Requena L. Extramammary Paget’s disease of the groin with underlying carcinoma and fatal outcome. Clin Exp Dermatol. 2008; 33:595–8.
Article15. Hurt MA, Hardarson S, Stadecker MJ, Santa Cruz DJ. Fibroepithelioma-like changes associated with anogenital epidermotropic mucinous carcinoma: fibroepitheliomatous Paget phenomenon. J Cutan Pathol. 1992; 19:134–41.
Article16. Taniyama K, Suzuki H, Yamada A, Tahara E. Extramammary Paget’s disease with an underlying mucinous adenocarcinoma of the buttocks. Gan No Rinsho. 1988; 34:379–84.17. Matin RN, Gibbon K, Rizvi H, Harwood CA, Cerio R. Cutaneous mucinous carcinoma arising in extramammary Paget disease of the perineum. Am J Dermatopathol. 2011; 33:705–9.
Article18. Bowling JC, Powles A, Nasiri N, Searle A, Bunker CB. Spontaneous regression of extramammary Paget's disease after excision of primary apocrine carcinoma, in an immunosuppressed patient. Br J Dermatol. 2005; 153:676–7.
Article19. Hernandez JM, Copeland EM 3rd. Infiltrating apocrine adenocarcinoma with extramammary pagetoid spread. Am Surg. 2007; 73:307–9.
Article20. Seo SH, Shin DH, Sung HW. Apocrine carcinoma of the groin possibly associated with extramammary Paget’s disease. Ann Dermatol. 2011; 23:519–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extramammary Paget's Disease of the Penis and Scrotum with a Renal Cell Carcinoma
- A Case of Cutaneous Mucinous Eccrine Carcinoma
- A Case of Cutaneous Mucinous Eccrine Carcinoma
- Giant Basal Cell Carcinoma Mimicking Extramammary Paget’s Disease
- Invasive Extramammary Paget Disease: A Report of 2 Cases with Immunohistochemical and Ultrastructural Findings