J Vet Sci.
2018 Jul;19(4):536-542. 10.4142/jvs.2018.19.4.536.
A Salmonella Typhi ghost induced by the E gene of phage φX174 stimulates dendritic cells and efficiently activates the adaptive immune response
- Affiliations
-
- 1College of Veterinary Medicine, Chonbuk National University, Iksan Campus, Iksan 54596, Korea. johnhlee@jbnu.ac.kr
- KMID: 2417569
- DOI: http://doi.org/10.4142/jvs.2018.19.4.536
Abstract
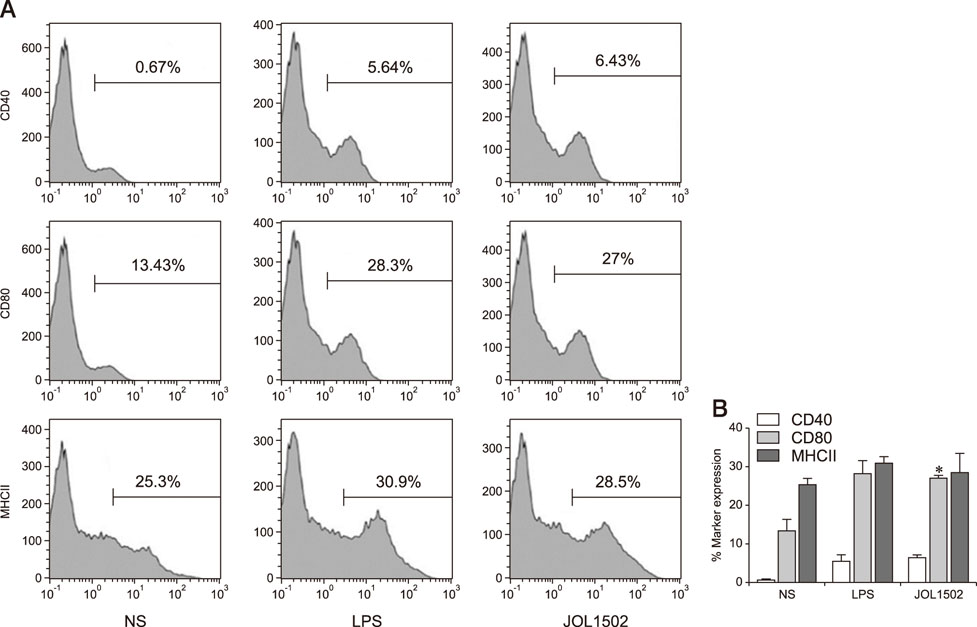
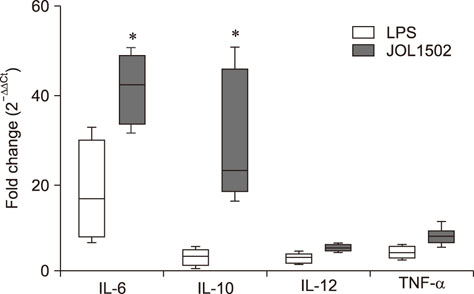
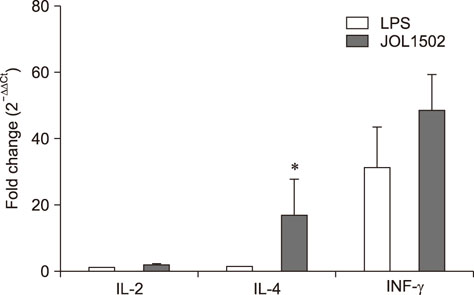
- Previously, we genetically engineered a Salmonella Typhi bacterial ghost (STG) as a novel inactivated vaccine candidate against typhoid fever. The underlying mechanism employed by the ghost in stimulating the adaptive immune response remains to be investigated. In this study, we aimed to evaluate the immunostimulatory effect of STG on mouse bone marrow-derived dendritic cells (BMDCs) and its activation of the adaptive immune response in vitro. Immature BMDCs were stimulated with STG, which efficiently stimulated maturation events in BMDCs, as indicated by upregulated expressions of CD40, CD80, and major histocompatibility complex class II molecules on CD11⺠BMDCs. Immature BMDCs responded to STG stimulation by significantly increasing the expression of interleukin (IL)-6, which might indicate the induction of dendritic cell maturation in vivo (p < 0.05). In addition, ghost-stimulated murine BMDCs showed significant expressions of interferon gamma and IL-4, which can drive the development of Th1 and Th2 cells, respectively, in co-cultured CD4⺠T cells in vitro. These results suggest that STG can effectively stimulate maturation of BMDCs and facilitate subsequent immune responses via potent immunomodulatory cytokine responses.
Keyword
MeSH Terms
Figure
Reference
-
1. Anwar E, Goldberg E, Fraser A, Acosta CJ, Paul M, Leibovici L. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev. 2014; CD001261.
Article2. Bermudez-Brito M, Muñoz-Quezada S, Gomez-Llorente C, Matencio E, Bernal MJ, Romero F, Gil A. Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS One. 2013; 8:e59370.3. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004; 82:346–353.4. de Jong HK, Parry CM, van der Poll T, Wiersinga WJ. Host-pathogen interaction in invasive Salmonellosis. PLoS Pathog. 2012; 8:e1002933.
Article5. Eko FO, Mania-Pramanik J, Pais R, Pan Q, Okenu DM, Johnson A, Ibegbu C, He C, He Q, Russell R, Black CM, Igietseme JU. Vibrio cholerae ghosts (VCG) exert immunomodulatory effect on dendritic cells for enhanced antigen presentation and induction of protective immunity. BMC Immunol. 2014; 15:584.
Article6. Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989; 170:2081–2095.
Article7. Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol. 2014; 5:391.
Article8. Garmory HS, Brown KA, Titball RW. Salmonella vaccines for use in humans: present and future perspectives. FEMS Microbiol Rev. 2002; 26:339–353.
Article9. Hajam IA, Dar PA, Appavoo E, Kishore S, Bhanuprakash V, Ganesh K. Bacterial ghosts of Escherichia coli drive efficient maturation of Bovine Monocyte-Derived Dendritic Cells. PLoS One. 2015; 10:e0144397.10. Hajam IA, Dar PA, Won G, Lee JH. Bacterial ghosts as adjuvants: mechanisms and potential. Vet Res. 2017; 48:37.
Article11. Haslberger AG, Kohl G, Felnerova D, Mayr UB, Fürst-Ladani S, Lubitz W. Activation, stimulation and uptake of bacterial ghosts in antigen presenting cells. J Biotechnol. 2000; 83:57–66.
Article12. Hur J, Lee JH. A new enterotoxigenic Escherichia coli vaccine candidate constructed using a Salmonella ghost delivery system: comparative evaluation with a commercial vaccine for neonatal piglet colibacillosis. Vet Immunol Immunopathol. 2015; 164:101–109.
Article13. Jawale CV, Chaudhari AA, Lee JH. Generation of a safety enhanced Salmonella Gallinarum ghost using antibiotic resistance free plasmid and its potential as an effective inactivated vaccine candidate against fowl typhoid. Vaccine. 2014; 32:1093–1099.
Article14. Jawale CV, Lee JH. Development of a biosafety enhanced and immunogenic Salmonella Enteritidis ghost using an antibiotic resistance gene free plasmid carrying a bacteriophage lysis system. PLoS One. 2013; 8:e78193.15. Kim B, Won G, Lee JH. Construction of an inactivated typhoid vaccine candidate expressing Escherichia coli heat-labile enterotoxin B subunit and evaluation of its immunogenicity in a murine model. J Med Microbiol. 2017; 66:1235–1243.
Article16. Kudela P, Paukner S, Mayr UB, Cholujova D, Schwarczova Z, Sedlak J, Bizik J, Lubitz W. Bacterial ghosts as novel efficient targeting vehicles for DNA delivery to the human monocyte-derived dendritic cells. J Immunother. 2005; 28:136–143.
Article17. Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W. The bacterial ghost platform system: production and applications. Bioeng Bugs. 2010; 1:326–336.18. Lin FY, Vo AH, Phan VB, Nguyen TT, Bryla D, Tran CT, Ha BK, Dang DT, Robbins JB. The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am J Trop Med Hyg. 2000; 62:644–648.
Article19. Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999; 223:77–92.
Article20. Nielsen KN, Steffensen MA, Christensen JP, Thomsen AR. Priming of CD8 T cells by adenoviral vectors is critically dependent on B7 and dendritic cells but only partially dependent on CD28 ligation on CD8 T cells. J Immunol. 2014; 193:1223–1232.
Article21. Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999; 11:305–312.
Article22. Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, Murakami M, Hirano T. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004; 173:3844–3854.
Article23. Sanders CJ, Yu Y, Moore DA 3rd, Williams IR, Gewirtz AT. Humoral immune response to flagellin requires T cells and activation of innate immunity. J Immunol. 2006; 177:2810–2818.
Article24. Seder RA, Hill AV. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000; 406:793–798.
Article25. Sojka DK, Bruniquel D, Schwartz RH, Singh NJ. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol. 2004; 172:6136–6143.
Article26. Srikanth CV, Mercado-Lubo R, Hallstrom K, McCormick BA. Salmonella effector proteins and host-cell responses. Cell Mol Life Sci. 2011; 68:3687–3697.27. Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006; 311:17–58.
Article28. Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002; 109:1519–1526.
Article29. Thoma-Uszynski S, Kiertscher SM, Ochoa MT, Bouis DA, Norgard MV, Miyake K, Godowski PJ, Roth MD, Modlin RL. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol. 2000; 165:3804–3810.
Article30. Wick MJ. Living in the danger zone: innate immunity to Salmonella. Curr Opin Microbiol. 2004; 7:51–57.31. Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann VS, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997; 185:317–328.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Typing of Salmonella typhi by Random Amplified Polymorphic DNA Analysis
- Salmonella Typhi Osteomyelitis in a Non-sickle Cell Patient: Three Cases Report
- Regulation of Intestinal Immune System by Dendritic Cells
- Molecular Analysis of Salmonella enterica Serotype typhi Isolated Sporadically in Seoul City
- Detection od salmonella typhi by polymerase chain reaction