J Vet Sci.
2018 Jul;19(4):512-518. 10.4142/jvs.2018.19.4.512.
Sciatic nerve leachate of cattle causes neuronal differentiation of PC12 cells via ERK1/2 signaling pathway
- Affiliations
-
- 1College of Animal Science and Technology, Henan University of Science and Technology, Luoyang, Henan 471023, China. lymzq@126.com
- KMID: 2417566
- DOI: http://doi.org/10.4142/jvs.2018.19.4.512
Abstract
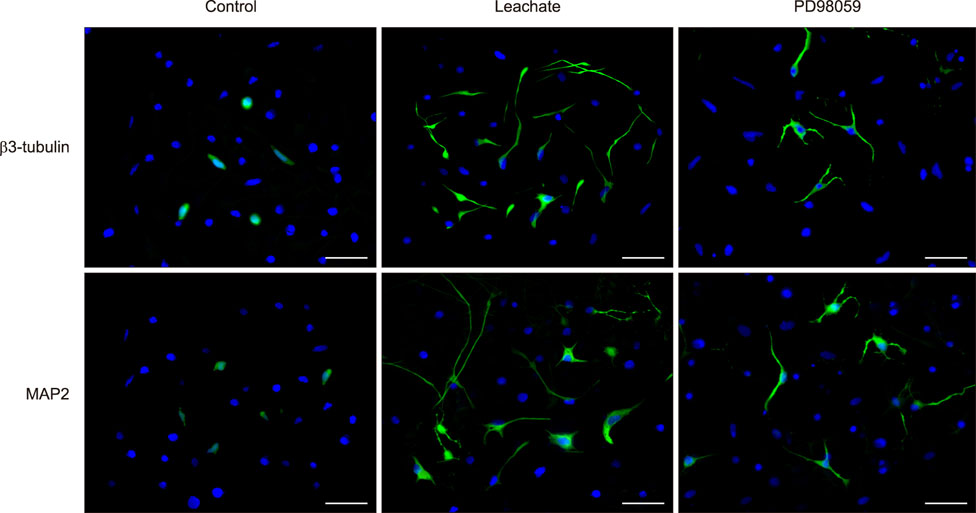
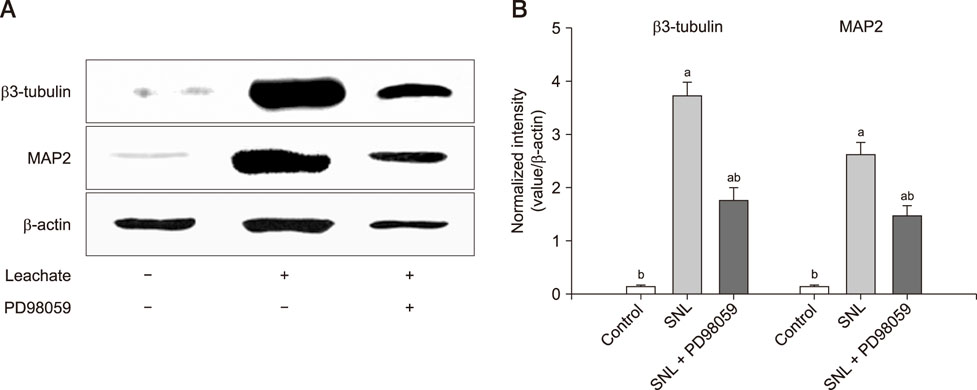
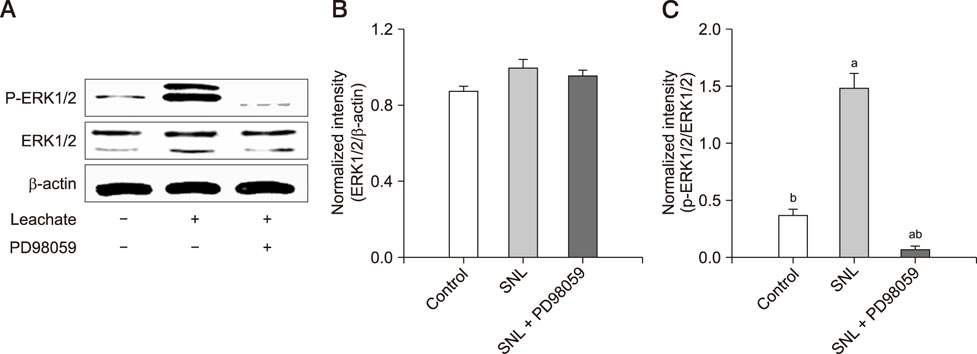
- Previous studies have shown that the sciatic nerve has neurotrophic activity, and nerve regeneration, differentiation, and axon outgrowth can be modulated by different sciatic nerve preparations. However, numerous animals may have to be sacrificed to obtain enough sciatic nerves to make a sciatic nerve preparation. Some studies have demonstrated that the role of sciatic nerve preparations in neural differentiation depends on the neurotrophins that Schwann cells secrete, and these factors are highly conserved among different species. To reduce the use of experimental animals, in this study, we made a leachate by using the sciatic nerve of cattle and explored its effect on neuronal differentiation of rat PC12 cells (a useful model for studying neuronal differentiation). Results showed the neurite outgrowth of PC12 cells treated with the cattle sciatic nerve leachate for 3, 6, and 9 days was significantly improved, and the expressions of β3-tubulin and microtubule-associated protein 2 (two neuron-specific proteins) were increased. Moreover, the ERK1/2 signaling pathway was activated after PC12 cells were incubated with cattle sciatic nerve leachate for 9 days. Thus, a sciatic nerve leachate obtained from cattle can effectively induce neuronal differentiation of rat PC12 cells via ERK1/2 signaling pathway.
Keyword
MeSH Terms
Figure
Reference
-
1. Bates DJ, Mangelsdorf DC, Ridings JA. Multiple neurotrophic factors including NGF-like activity in nerve regeneration chamber fluids. Neurochem Int. 1995; 26:281–293.
Article2. Castillo C, Carreño F, Villegas GM, Villegas R. Ionic currents in PC12 cells differentiated into neuron-like cells by a cultured-sciatic nerve conditioned medium. Brain Res. 2001; 911:181–192.
Article3. Eggers R, Tannemaat MR, Ehlert EM, Verhaagen J. A spatio-temporal analysis of motoneuron survival, axonal regeneration and neurotrophic factor expression after lumbar ventral root avulsion and implantation. Exp Neurol. 2010; 223:207–220.
Article4. Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003; 23:1416–1423.
Article5. Guo C, Hou J, Ao S, Deng X, Lyu G. HOXC10 up-regulation promotes gastric cancer cell proliferation and metastasis through MAPK pathway. Chin J Cancer Res. 2017; 29:572–580.
Article6. Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP, Berkemeier L, Phillips HS, Rosenthal A. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993; 363:266–270.
Article7. Howe CL. Depolarization of PC12 cells induces neurite outgrowth and enhances nerve growth factor-induced neurite outgrowth in rats. Neurosci Lett. 2003; 351:41–45.
Article8. Kuffler DP, Megwinoff O. Neurotrophic influence of denervated sciatic nerve on adult dorsal root ganglion neurons. J Neurobiol. 1994; 25:1267–1282.
Article9. Li R, Ma J, Wu Y, Nangle M, Zou S, Li Y, Yin J, Zhao Y, Xu H, Zhang H, Li X, Ye QS, Wang J, Xiao J. Dual delivery of NGF and bFGF coacervater ameliorates diabetic peripheral neuropathy via inhibiting Schwann cells apoptosis. Int J Biol Sci. 2017; 13:640–651.
Article10. Lin JY, Wu CL, Liao CN, Higuchi A, Ling QD. Chemogenomic analysis of neuronal differentiation with pathway changes in PC12 cells. Mol Biosyst. 2016; 12:283–294.
Article11. Liu Y, Zhang Z, Lv Q, Chen X, Deng W, Shi K, Pan L. Effects and mechanisms of melatonin on the proliferation and neural differentiation of PC12 cells. Biochem Biophys Res Commun. 2016; 478:540–545.
Article12. Liu Y, Zhang Z, Qin Y, Wu H, Lv Q, Chen X, Deng W. A new method for Schwann-like cell differentiation of adipose derived stem cells. Neurosci Lett. 2013; 551:79–83.
Article13. Liu Z, Zhu S, Liu L, Ge J, Huang L, Sun Z, Zeng W, Huang J, Luo Z. A magnetically responsive nanocomposite scaffold combined with Schwann cells promotes sciatic nerve regeneration upon exposure to magnetic field. Int J Nanomedicine. 2017; 12:7815–7832.
Article14. Lu X, Zhang N, Dong S, Hu Y. Involvement of GPR12 in the induction of neurite outgrowth in PC12 cells. Brain Res Bull. 2012; 87:30–36.
Article15. Luo B, Huang J, Lu L, Hu X, Luo Z, Li M. Electrically induced brain-derived neurotrophic factor release from Schwann cells. J Neurosci Res. 2014; 92:893–903.
Article16. Malavé C, Villegas GM, Hernández M, Martínez JC, Castillo C, Suárez de Mata Z, Villegas R. Role of glypican-1 in the trophic activity on PC12 cells induced by cultured sciatic nerve conditioned medium: identification of a glypican-1-neuregulin complex. Brain Res. 2003; 983:74–83.
Article17. Morrison RS, Keating RF, Moskal JR. Basic fibroblast growth factor and epidermal growth factor exert differential trophic effects on CNS neurons. J Neurosci Res. 1988; 21:71–79.
Article18. Nabiuni M, Rasouli J, Parivar K, Kochesfehani HM, Irian S, Miyan JA. In vitro effects of fetal rat cerebrospinal fluid on viability and neuronal differentiation of PC12 cells. Fluids Barriers CNS. 2012; 9:8.
Article19. Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment--implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007; 59:1329–1339.
Article20. Shakhbazau A, Martinez JA, Xu QG, Kawasoe J, van Minnen J, Midha R. Evidence for a systemic regulation of neurotrophin synthesis in response to peripheral nerve injury. J Neurochem. 2012; 122:501–511.
Article21. Soltani MH, Pichardo R, Song Z, Sangha N, Camacho F, Satyamoorthy K, Sangueza OP, Setaluri V. Microtubule-associated protein 2, a marker of neuronal differentiation, induces mitotic defects, inhibits growth of melanoma cells, and predicts metastatic potential of cutaneous melanoma. Am J Pathol. 2005; 166:1841–1850.
Article22. Srivastava A, Singh S, Pandey A, Kumar D, Rajpurohit CS, Khanna VK, Pant AB. Secretome of differentiated PC12 cells enhances neuronal differentiation in human mesenchymal stem cells via NGF-like mechanism. Mol Neurobiol. 2018; Epub ahead of print. DOI: 10.1007/s12035-018-0981-4.
Article23. Tsai TH, Tai CC, Wu YHS, Hung SH, Chang SF, Yang CP, Tseng H. Gene expression and behavior analysis of PC12 cells grown on synthetic biodegradable fibrous membranes coated with natural biopolymers. Curr Nanosci. 2014; 10:235–242.
Article24. Villegas R, Villegas GM, Longart M, Hernández M, Maqueira B, Buonanno A, García R, Castillo C. Neuregulin found in cultured-sciatic nerve conditioned medium causes neuronal differentiation of PC12 cells. Brain Res. 2000; 852:305–318.
Article25. Wade A, Thomas C, Kalmar B, Terenzio M, Garin J, Greensmith L, Schiavo G. Activated leukocyte cell adhesion molecule modulates neurotrophin signaling. J Neurochem. 2012; 121:575–586.
Article26. Won JH, Ahn KH, Back MJ, Ha HC, Jang JM, Kim HH, Choi SZ, Son M, Kim DK. DA-9801 promotes neurite outgrowth via ERK1/2-CREB pathway in PC12 cells. Biol Pharm Bull. 2015; 38:169–178.
Article27. Wu SD, Xia F, Lin XM, Duan KL, Wang F, Lu QL, Cao H, Qian YH, Shi M. Ginsenoside-Rd promotes neurite outgrowth of PC12 cells through MAPK/ERK- and PI3K/AKT-dependent pathways. Int J Mol Sci. 2016; 17:177.
Article28. Yamauchi N, Taguchi Y, Kato H, Umeda M. High-power, red-light-emitting diode irradiation enhances proliferation, osteogenic differentiation, and mineralization of human periodontal ligament stem cells via ERK signaling pathway. J Periodontol. 2018; 89:351–360.
Article29. Ye Q, Zhang X, Huang B, Zhu Y, Chen X. Astaxanthin suppresses MPP+-induced oxidative damage in PC12 cells through a Sp1/NR1 signaling pathway. Mar Drugs. 2013; 11:1019–1034.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aucubin Promotes Neurite Outgrowth in Neural Stem Cells and Axonal Regeneration in Sciatic Nerves
- Effect of Extracellular Signal-Regulated Kinase Inhibition on Oxysterol 7-Ketocholesterol-Induced Apoptosis
- Calcium overload is essential for the acceleration of staurosporine-induced cell death following neuronal differentiation in PC12 cells
- Involvement of Endoplasmic Reticulum Stress Response in the Neuronal Differentiation
- Neuroprotection Signaling of Nuclear Akt in Neuronal Cells