J Korean Neurosurg Soc.
2018 May;61(3):386-392. 10.3340/jkns.2018.0004.
Radiation Therapy against Pediatric Malignant Central Nervous System Tumors : Embryonal Tumors and Proton Beam Therapy
- Affiliations
-
- 1Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dh8lim@skku.edu
- KMID: 2417363
- DOI: http://doi.org/10.3340/jkns.2018.0004
Abstract
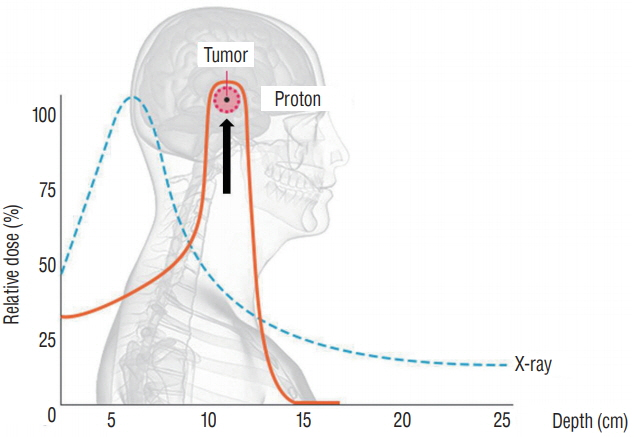
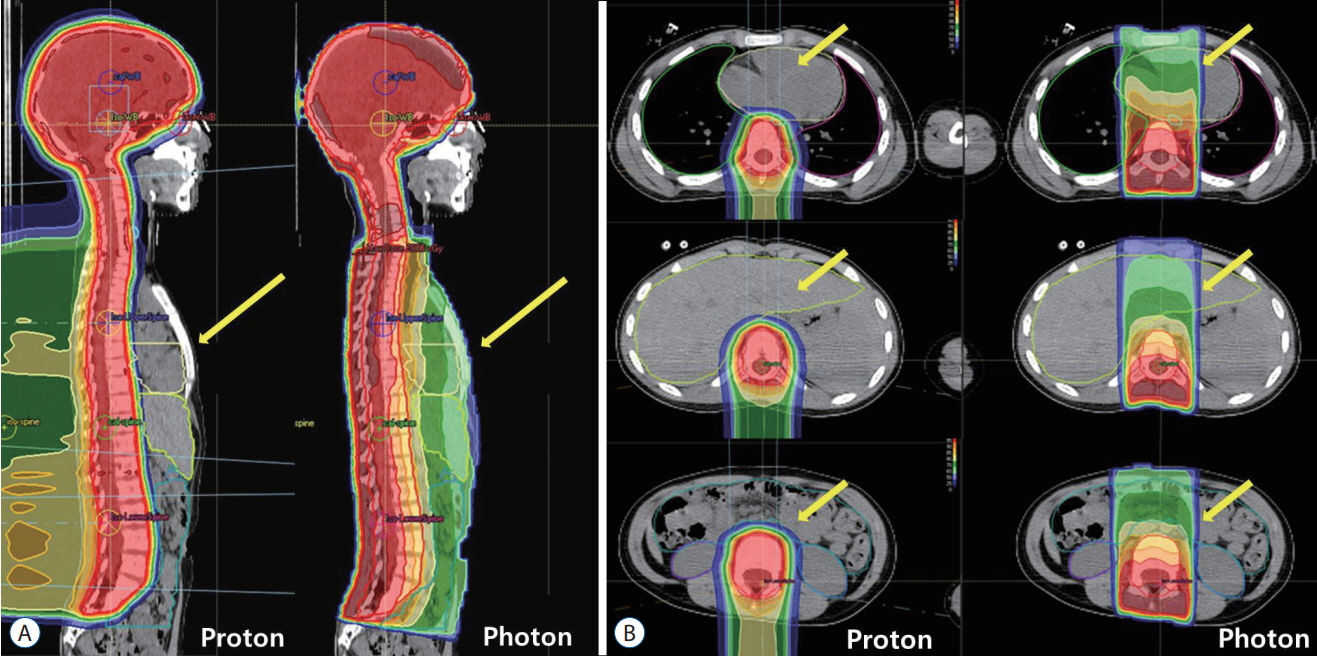
- Radiation therapy is highly effective for the management of pediatric malignant central nervous system (CNS) tumors including embryonal tumors. With the increment of long-term survivors from malignant CNS tumors, the radiation-related toxicities have become a major concern and we need to improve the treatment strategies to reduce the late complications without compromising the treatment outcomes. One of such strategies is to reduce the radiation dose to craniospinal axis or radiation volume and to avoid or defer radiation therapy until after the age of three. Another strategy is using particle beam therapy such as proton beams instead of photon beams. Proton beams have distinct physiologic advantages over photon beams and greater precision in radiation delivery to the tumor while preserving the surrounding healthy tissues. In this review, I provide the treatment principles of pediatric CNS embryonal tumors and the strategic improvements of radiation therapy to reduce treatment-related late toxicities, and finally introduce the increasing availability of proton beam therapy for pediatric CNS embryonal tumors compared with photon beam therapy.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Armstrong GT. Long-term survivors of childhood central nervous system malignancies: the experience of the Childhood Cancer Survivor Study. Eur J Paediatr Neurol. 14:298–303. 2010.
Article2. Chi SN, Gardner SL, Levy AS, Knopp EA, Miller DC, Wisoff JH, et al. Feasibility and response to induction chemotherapy intensified with highdose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol. 22:4881–4887. 2004.
Article3. Chung CS, Yock TI, Nelson K, Xu Y, Keating NL, Tarbell NJ. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 87:46–52. 2013.
Article4. Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin-Lottick NS, Kawashima TI, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 27:2339–2355. 2009.
Article5. Fangusaro J, Finlay J, Sposto R, Ji L, Saly M, Zacharoulis S, et al. Intensive chemotherapy followed by consolidative myeloablative chemotherapy with autologous hematopoietic cell rescue (AuHCR) in young children with newly diagnosed supratentorial primitive neuroectodermal tumors (sPNETs): report of the Head Start I and II experience. Pediatr Blood Cancer. 50:312–318. 2008.
Article6. Grill J, Renaux VK, Bulteau C, Viguier D, Levy-Piebois C, Sainte-Rose C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 45:137–145. 1999.
Article7. Kortmann RD, Kühl J, Timmermann B, Mittler U, Urban C, Budach V, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT ‘91. Int J Radiat Oncol Biol Phys. 46:269–279. 2000.
Article8. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114:97–109. 2007.
Article9. Marachelian A, Butturini A, Finlay J. Myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for childhood central nervous system tumors. Bone Marrow Transplant. 41:167–172. 2008.
Article10. Mason WP, Grovas A, Halpern S, Dunkel IJ, Garvin J, Heller G, et al. Intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. J Clin Oncol. 16:210–221. 1998.
Article11. Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cisretinoic acid: a children’s oncology group study. J Clin Oncol. 27:1007–1013. 2009.
Article12. Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 51:110–117. 2008.
Article13. Merchant TE, Kun LE, Krasin MJ, Wallace D, Chintagumpala MM, Woo SY, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys. 70:782–787. 2008.
Article14. Moxon-Emre I, Bouffet E, Taylor MD, Laperriere N, Scantlebury N, Law N, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 32:1760–1768. 2014.
Article15. Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol. 16:1723–1728. 1998.
Article16. Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 5:399–408. 2004.
Article17. Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 355:1572–1582. 2006.
Article18. Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 24:4202–4208. 2006.
Article19. Packer RJ, Gurney JG, Punyko JA, Donaldson SS, Inskip PD, Stovall M, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 21:3255–3261. 2003.
Article20. Packer RJ, Rood BR, MacDonald TJ. Medulloblastoma: present concepts of stratification into risk groups. Pediatr Neurosurg. 39:60–67. 2003.
Article21. Park ES, Sung KW, Baek HJ, Park KD, Park HJ, Won SC, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in young children with atypical teratoid/rhabdoid tumor of the central nervous system. J Korean Med Sci. 27:135–140. 2012.
Article22. Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 19:3470–3476. 2001.
Article23. Sung KW, Lim DH, Son MH, Lee SH, Yoo KH, Koo HH, et al. Reduceddose craniospinal radiotherapy followed by tandem high-dose chemotherapy and autologous stem cell transplantation in patients with highrisk medulloblastoma. Neuro Oncol. 15:352–359. 2013.
Article24. Sung KW, Lim DH, Yi ES, Choi YB, Lee JW, Yoo KH, et al. Tandem highdose chemotherapy and autologous stem cell transplantation for atypical teratoid/rhabdoid Tumor. Cancer Res Treat. 48:1408–1419. 2016.
Article25. Taylor RE, Bailey CC, Robinson KJ, Weston CL, Walker DA, Ellison D, et al. Outcome for patients with metastatic (M2-3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur J Cancer. 41:727–734. 2005.
Article26. Tekautz TM, Fuller CE, Blaney S, Fouladi M, Broniscer A, Merchant TE, et al. Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol. 23:1491–1499. 2005.
Article27. Thomas PR, Deutsch M, Kepner JL, Boyett JM, Krischer J, Aronin P, et al. Low-stage medulloblastoma: final analysis of trial comparing standarddose with reduced-dose neuraxis irradiation. J Clin Oncol. 18:3004–3011. 2000.
Article28. Woehrer A, Slavc I, Waldhoer T, Heinzl H, Zielonke N, Czech T, et al. Incidence of atypical teratoid/rhabdoid tumors in children: a populationbased study by the Austrian Brain Tumor Registry, 1996-2006. Cancer. 116:5725–5732. 2010.
Article29. Wolden SL, Dunkel IJ, Souweidane MM, Happersett L, Khakoo Y, Schupak K, et al. Patterns of failure using a conformal radiation therapy tumor bed boost for medulloblastoma. J Clin Oncol. 21:3079–3083. 2003.
Article30. Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 17:832–845. 1999.
Article31. Zhang R, Howell RM, Taddei PJ, Giebeler A, Mahajan A, Newhauser WD. A comparative study on the risks of radiogenic second cancers and cardiac mortality in a set of pediatric medulloblastoma patients treated with photon or proton craniospinal irradiation. Radiother Oncol. 113:84–88. 2014.
Article