J Korean Neurosurg Soc.
2016 Nov;59(6):570-576. 10.3340/jkns.2016.59.6.570.
Retrospective Analysis of Cerebrospinal Fluid Profiles in 228 Patients with Leptomeningeal Carcinomatosis : Differences According to the Sampling Site, Symptoms, and Systemic Factors
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of System Cancer Science, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea. nsghs@ncc.re.kr
- 3Cancer Biostatistics Branch, National Cancer Center, Goyang, Korea.
- 4Neuro-Oncology Clinic, National Cancer Center, Goyang, Korea.
- KMID: 2417338
- DOI: http://doi.org/10.3340/jkns.2016.59.6.570
Abstract
OBJECTIVE
Elevated cell counts and protein levels in cerebrospinal fluid (CSF) result from disease activity in patients with leptomeningeal carcinomatosis (LMC). Previous studies evaluated the use of CSF profiles to monitor a treatment response or predict prognosis. CSF profiles vary, however, according to the sampling site and the patient's systemic condition. We compared lumbar and ventricular CSF profiles collected before intraventricular chemotherapy for LMC and evaluated the association of these profiles with patients' systemic factors and LMC disease activity.
METHODS
CSF profiles were retrospectively collected from 228 patients who underwent Ommaya reservoir insertion for intraventricular chemotherapy after a diagnosis of LMC. Lumbar samples taken via lumbar puncture were used for the diagnosis, and ventricular samples were obtained later at the time of Ommaya reservoir insertion. LMC disease activity was defined as the presence of LMC-related symptoms such as increased intracranial pressure, hydrocephalus, cranial neuropathy, and cauda equina syndrome.
RESULTS
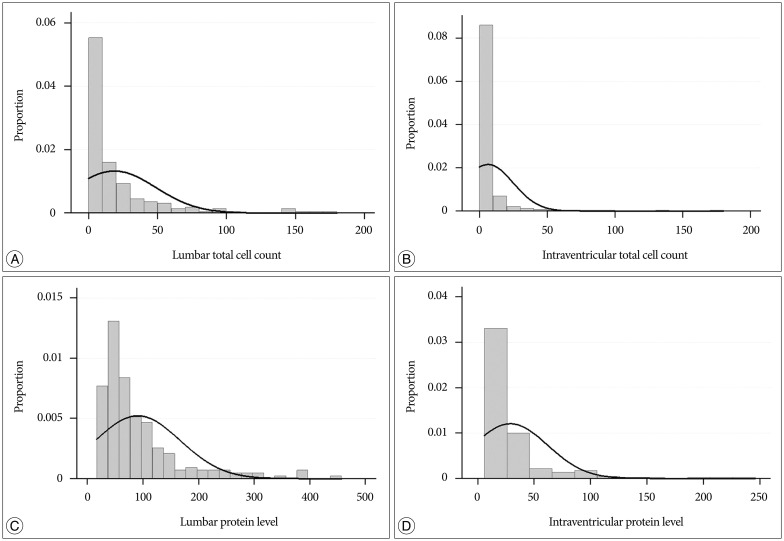
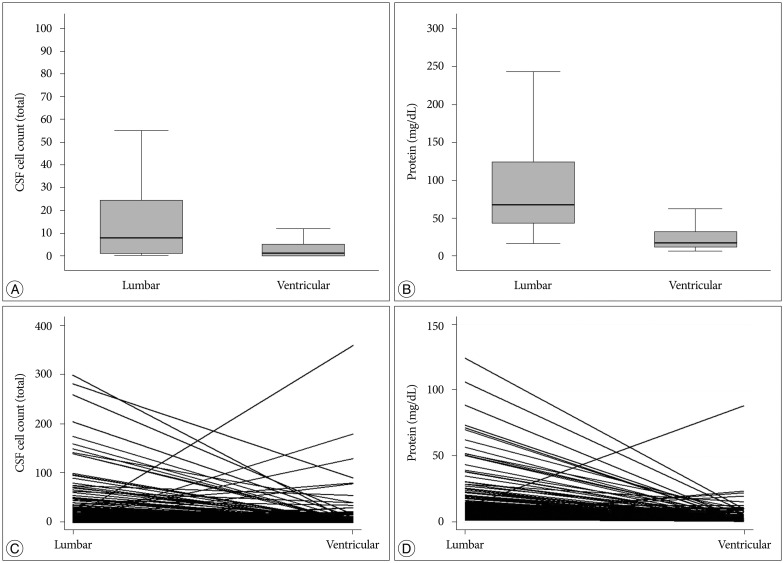
Cell counts (median : 8 vs. 1 cells/mL) and protein levels (median : 68 vs. 17 mg/dL) significantly higher in lumbar CSF than in ventricular CSF (p<0.001). Among the evaluated systemic factors, concomitant brain metastasis and previous radiation were significantly correlated with higher protein levels in the lumbar CSF (p=0.01 and <0.001, respectively). Among the LMC disease activity, patients presenting with hydrocephalus or cauda equina syndrome showed higher lumbar CSF protein level compared with that in patients without those symptoms (p=0.049 and p<0.001, respectively). The lumbar CSF cell count was significantly lower in patients with cranial neuropathy (p=0.046). The ventricular CSF cell counts and protein levels showed no correlation with LMC symptoms. Carcinoembryonic antigen (CEA), which was measured from ventricular CSF after the diagnosis in 109 patients, showed a significant association with the presence of hydrocephalus (p=0.01).
CONCLUSION
The protein level in lumbar CSF indicated the localized disease activity of hydrocephalus and cauda equina syndrome. In the ventricular CSF, only the CEA level reflected the presence of hydrocephalus. We suggest using more specific biomarkers for the evaluation of ventricular CSF to monitor disease activity and treatment response.
MeSH Terms
Figure
Cited by 1 articles
-
Cerebrospinal Fluid Profiles and Their Changes after Intraventricular Chemotherapy as Prognostic or Predictive Markers for Patients with Leptomeningeal Carcinomatosis
Ji-Woong Kwon, Youngbo Shim, Ho-Shin Gwak, Eun Young Park, Jungnam Joo, Heon Yoo, Sang-Hoon Shin
J Korean Neurosurg Soc. 2021;64(4):631-643. doi: 10.3340/jkns.2020.0300.
Reference
-
1. An YJ, Cho HR, Kim TM, Keam B, Kim JW, Wen H, et al. An NMR metabolomics approach for the diagnosis of leptomeningeal carcinomatosis in lung adenocarcinoma cancer patients. Int J Cancer. 2015; 136:162–171. PMID: 24798643.
Article2. Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996; 53:626–632. PMID: 8929170.3. Boogerd W, Hart AA, van der Sande JJ, Engelsman E. Meningeal carcinomatosis in breast cancer. Prognostic factors and influence of treatment. Cancer. 1991; 67:1685–1695. PMID: 2001559.
Article4. Bruna J, González L, Miró J, Velasco R, Gil M, Tortosa A;. Leptomeningeal carcinomatosis : prognostic implications of clinical and cerebrospinal fluid features. Cancer. 2009; 115:381–389. PMID: 19109820.5. Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol. 2010; 22:627–635. PMID: 20689429.
Article6. Chamberlain MC. Radioisotope CSF flow studies in leptomeningeal metastases. J Neurooncol. 1998; 38:135–140. PMID: 9696363.7. Chamberlain MC, Kormanik PA, Glantz MJ. A comparison between ventricular and lumbar cerebrospinal fluid cytology in adult patients with leptomeningeal metastases. Neuro Oncol. 2001; 3:42–45. PMID: 11305416.
Article8. Clamon G, Doebbeling B. Meningeal carcinomatosis from breast cancer : spinal cord vs. brain involvement. Breast Cancer Res Treat. 1987; 9:213–217. PMID: 3663957.
Article9. Fizazi K, Asselain B, Vincent-Salomon A, Jouve M, Dieras V, Palangie T, et al. Meningeal carcinomatosis in patients with breast carcinoma. Clinical features, prognostic factors, and results of a high-dose intrathecal methotrexate regimen. Cancer. 1996; 77:1315–1323. PMID: 8608509.
Article10. Glantz MJ, Hall WA, Cole BF, Chozick BS, Shannon CM, Wahlberg L, et al. Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer. 1995; 75:2919–2931. PMID: 7773943.
Article11. Glass JP, Melamed M, Chernik NL, Posner JB. Malignant cells in cerebrospinal fluid (CSF) the meaning of a positive CSF cytology. Neurology. 1979; 29:1369–1375. PMID: 573381.
Article12. Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999; 25:103–119. PMID: 10395835.
Article13. Gwak HS, Lee CH, Yang HS, Joo J, Shin SH, Yoo H, et al. Chemoport with a non-collapsible chamber as a replacement for an Ommaya reservoir in the treatment of leptomeningeal carcinomatosis. Acta Neurochir (Wien). 2011; 153:1971–1978. discussion 1978. PMID: 21796363.
Article14. Herrlinger U, Förschler H, Küker W, Meyermann R, Bamberg M, Dichgans J, et al. Leptomeningeal metastasis : survival and prognostic factors in 155 patients. J Neurol Sci. 2004; 223:167–178. PMID: 15337619.
Article15. Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol. 1987; 5:1655–1662. PMID: 3309199.
Article16. Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain : evaluation of the evidence. Fluids Barriers CNS. 2014; 11:26. PMID: 25678956.
Article17. Jayson GC, Howell A. Carcinomatous meningitis in solid tumours. Ann Oncol. 1996; 7:773–786. PMID: 8922190.
Article18. Kaplan JG, DeSouza TG, Farkash A, Shafran B, Pack D, Rehman F, et al. Leptomeningeal metastases : comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol. 1990; 9:225–229. PMID: 2086737.
Article19. Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases : proposal from the RANO group. Lancet Oncol. 2015; 16:e270–e278. PMID: 26065612.20. Nakagawa H, Kubo S, Murasawa A, Nakajima S, Nakajima Y, Izumoto S, et al. Measurements of CSF biochemical tumor markers in patients with meningeal carcinomatosis and brain tumors. J Neurooncol. 1992; 12:111–120. PMID: 1560255.
Article21. Park JH, Kim YJ, Lee JO, Lee KW, Kim JH, Bang SM, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. 2012; 76:387–392. PMID: 22186628.
Article22. Taillibert S, Laigle-Donadey F, Chodkiewicz C, Sanson M, Hoang-Xuan K, Delattre JY. Leptomeningeal metastases from solid malignancy : a review. J Neurooncol. 2005; 75:85–99. PMID: 16215819.
Article23. Teplyuk NM, Mollenhauer B, Gabriely G, Giese A, Kim E, Smolsky M, et al. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol. 2012; 14:689–700. PMID: 22492962.
Article24. Twijnstra A, Ongerboer de Visser BW, van Zanten AP. Diagnosis of leptomeningeal metastasis. Clin Neurol Neurosurg. 1987; 89:79–85. PMID: 3595019.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to “Retrospective Analysis of Cerebrospinal Fluid Profiles in 228 Patients with Leptomeningeal Carcinomatosis : Differences According to the Sampling Site, Symptoms, and Systemic Factors” by Shim Y, et al. (J Korean Neurosurg Soc 59 : 570-576, 2016)
- Chest Wall Pain as the Presenting Symptom of Leptomeningeal Carcinomatosis
- Response of Leptomeningeal Dissemination of Anaplastic Glioma to Temozolomide: Experience of Two Cases
- Cerebrospinal Fluid Profiles and Their Changes after Intraventricular Chemotherapy as Prognostic or Predictive Markers for Patients with Leptomeningeal Carcinomatosis
- A Retrospective Analysis of the Clinical Outcomes of Leptomeningeal Metastasis in Patients with Solid Tumors