J Korean Acad Conserv Dent.
2007 Nov;32(6):498-513.
The change of the configuration of hydroxyapatite crystals in enamel by changes of pH and degree of saturation of lactic acid buffer solution
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Yonsei University, Korea. chanyoungl@yumc.yonsei.ac.kr
Abstract
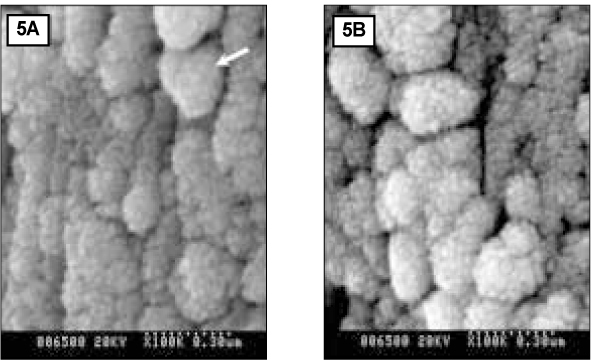
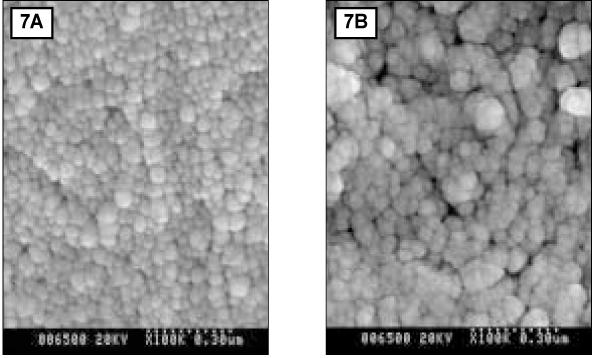
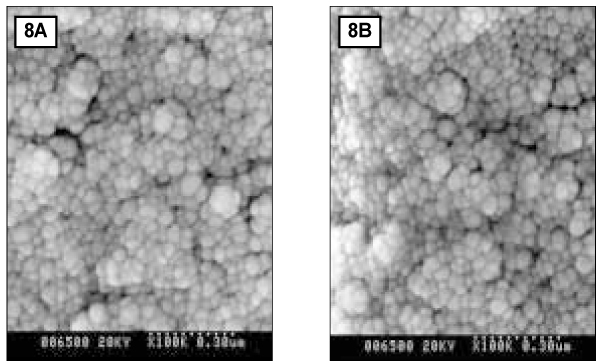
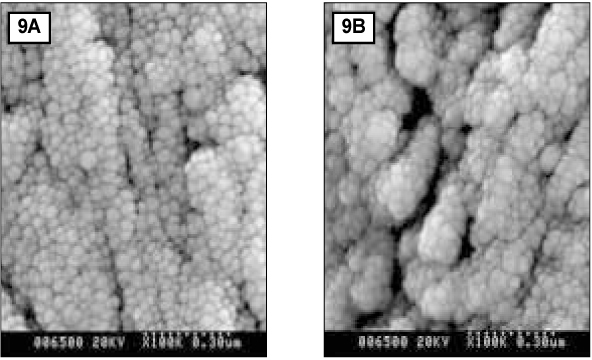
- Since it was reported that incipient enamel caries can be recovered, previous studies have quantitatively evaluated that enamel artificial caries have been remineralized with fluoride, showing simultaneously the increase of width of surface layer and the decrease of width of the body of legion. There is, however, little report which showed that remineralization could occur without fluoride. In addition, the observations on the change of hydroxyapatite crystals also have been scarcely seen. In this study, enamel caries in intact premolars or molars was induced by using lactic acidulated buffering solutions over 2 days. Then decalcified specimens were remineralized by seven groups of solutions using different degree of saturation (0.212, 0.239, 0.301, 0.355) and different pH (5.0, 5.5, 6.0) over 10 days. A qualitative comparison to changes of hydroxyapatite crystals after fracturing teeth was made under SEM (scanning electron microscopy) and AFM (atomic force microscopy). The results were as follows: 1. The size of hydroxyapatite crystals in demineralized area was smaller than the normal ones. While the space among crystals was expanded, it was observed that crystals are arranged irregularly. 2. In remineralized enamel area, the enlarged crystals with various shape were observed when the crystals were fused and new small crystals in intercrystalline spaces were deposited. 3. Group 3 and 4 with higher degree of saturation at same pH showed the formation of large clusters by aggregation of small crystals from the surface layer to the lesion body than group 1 and 2 with relatively low degree of saturation at same pH did. Especially group 4 showed complete remineralization to the body of lesions. Group 5 and 6 with lower pH at similar degree of saturation showed remineralization to the body of lesions while group 7 didn't show it. Unlike in Group 3 and 4, Group 5 and 6 showed that each particle was densely distributed with clear appearance rather than crystals form clusters together.
Keyword
MeSH Terms
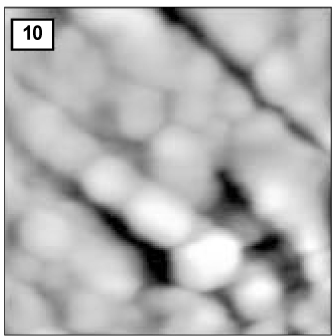
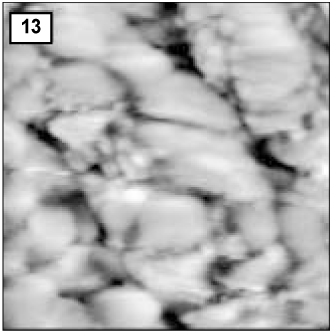
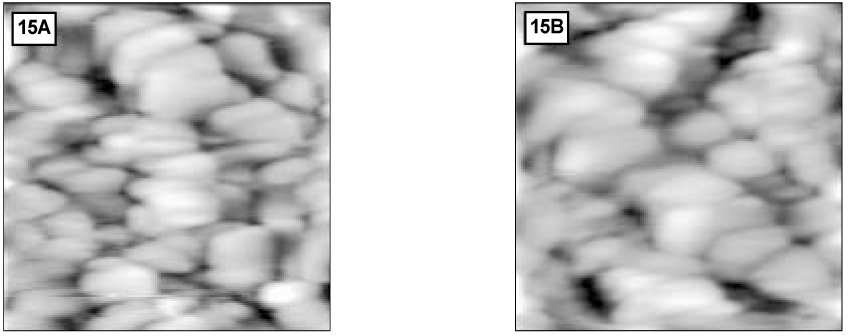
Figure
Reference
-
1. Aoba T. Solubility properties of human tooth mineral and pathogenesis of dental caries. Oral Dis. 2004. 10:249–257.
Article2. Aoba T, Moriwaki Y, Doi Y, Okazaki M, Takahasi J, Yagi T. The intact surface layer in natural enamel caries and acid dissolved hydroxyapatite pellets. J Oral Pathol. 1981. 10:32–39.
Article3. Applebaum E. Incipient dental caries. J Dent Res. 1932. 12:619–627.
Article4. Arends J, Jongebloed W, Ogaard B, Rolla G. SEM and microradiographic investigation of initial enamel caries. Scand J Dent Res. 1987. 95:193–201.
Article5. Areds J, Ten Cate JM. Tooth enamel remineralization. J Crystal Growth. 1981. 53:135–147.
Article6. Backer D. Posteruptive changes in dental enamel. J Dent Res. 1966. 45:503–511.
Article7. Brown WE, Chow LC. Thermodynamics of apatite crystal growth and dissolution. J Crystal Growth. 1981. 53:31–41.
Article8. Buskes JA, Christoffersen J, Arends J. Lesion formation and lesion remineralization in enamel under constant composition condition. Caries Res. 1985. 19:490–496.
Article9. Darling AI. Studies of the Early Lesion of Enamel Caries with Transmitted Light, Polarized Light and Microradiography. Its nature, mode of spread, points of entry and its relation to enamel structure. Br Dent J. 1958. 105:119–135.10. de Rooij JF, Nancollas GH. The formation and remineralization of artificial white spot lesions : a constant composition approach. J Dent Res. 1984. 63(6):864–867.
Article11. Exterkate RAM, Damen JJM, Ten Cate JM. A single-section model for enamel de- and remineralization studies. 1. The effects of different Ca/P ratios in remineralization solutions. J Dent Res. 1993. 72(12):1599–1603.
Article12. Feagin F, Patel PR, Koulourides T, Pigman W. Study of the effect of calcium, phosphate, fluoride and hydrogen ion concentrations on the remineralization of partially demineralized human and bovine enamel surfaces. Arch Oral Biol. 1971. 16:535–548.
Article13. Featherstone JD. The continuum of dental caries. - Evidence for a dynamic disease process. J Dent Res. 2004. 83 Spec No C:C39–C42.14. Featherstone JD, Mellberg JR. Relative rates of progress of artificial carious lesion in bovine, ovine and human enamel. Caries Res. 1981. 15:109–114.
Article15. Frank RM. Structural events in the caries process in enamel, cementum, and dentin. J Dent Res. 1990. 69 Spec No:599–566.
Article16. Gao XJ, Elliott JC, Anderson P. Scanning and contact microradiographic study of the effect of degree of saturation on the rate of enamel demineralization. J Dent Res. 1991. 70:1332–1337.
Article17. Gerdin PO. Studies in dentifrice. Clinical testing of an acidulated, nongrinding dentifrice with reduced fluoride contents. Sven Tandlak Tidskr. 1974. 67(5):283–297.18. Haikel Y, Frank RM, Voegel LC. Scanning electron microscopy of the human enamel surface layer of incipient carious lesions. Caries Res. 1983. 17:1–13.
Article19. Hallsworth AS, Weather JA, Robinson C. Loss of carbonate during the first stages of enamel caries. Caries Res. 1973. 7:345–348.
Article20. Hayashi Y. High resolution electron microscopy of enamel crystallite demineralized by initial dental caries. Scanning Microsc. 1995. 9:199–205.21. Head JA. A study of saliva and its action on tooth enamel in reference to its hardening and softening. JAMA. 1912. 59:2118–2122.
Article22. Holmen L, Thylstrup A, Featherstone JDB, Fredebo L, Shariati M. A scanning electron microscopic study of surface changes during development of artificial caries. Caries Res. 1985. 19:11–21.
Article23. Jacobeson APM, Strang R, Stephen KW. Effect of low fluoride levels in de/remineralising solutions of a pH-cycling model. Caries Res. 1991. 25:230–231.24. Johnson NW. Some aspects of the ultrastructure of early human caries seen with the electron microscope. Arch Oral Biol. 1967. 12:1505–1521.
Article25. Koch G, Petersson LG, Kling E. Effect of 250 and 1000ppm fluoride dentifrice on caries. A three-year clinical study. Swed Dent J. 1982. 6(6):233–238.26. Lammers PC, Borggreven JM, Driessens FC. Influence of fluoride on in vitro remineralization of artificial subsurface lesions determined with a sandwich technique. Caries Res. 1990. 24:81–85.
Article27. Lammers PC, Borggreven JM, Driessens FC. Influence of fluoride and pH on in vitro remineralization of bovine enamel. Caries Res. 1992. 26:8–13.
Article28. Margolis HC, Moreno EC. Kinetics and thermodynamic aspect of enamel demineralization. Caries Res. 1985. 19:22–35.
Article29. Margolis HC, Moreno EC. Physicochemical perspective on the cariostatic mechanism of systemic and topical fluorides. J Dent Res. 1990. 69 Spec No:606–613.30. Margolis HC, Zang YP, Lee CY, Kent RL, Moreno EC. Kinetics of enamel demineralization in vitro. J Dent Res. 1999. 78(7):1326–1335.31. Moreno EC, Margolis HC. Composition of human plaque fluid. J Dent Res. 1988. 67:1181–1189.
Article32. Moreno EC, Zahradnik RT. Chemistry of enamel subsurface demineralization in vitro. J Dent Res. 1974. 53(2):226–235.
Article33. Nancollas GH. Enamel apatite nucleation and crystal growth. J Dent Res. 1979. 58(Spec Issue B):861–870.
Article34. Pearce EI, Larsen MJ, Cutress TW. Studies on the influence of fluoride on the equilibrating calcium phosphate phase at a high enamel/acid ratio. Caries Res. 1995. 29:258–265.
Article35. Shellis RP. A scanning electron-microscopic study of solubility variations in human enamel and dentine. Arch Oral Biol. 1996. 41:473–495.
Article36. Shellis RP, Hallsworth AS. The use of scanning electron microscopy in studying enamel caries. Scanning Microsc. 1987. 1(3):1109–1123.37. Silverstone LM. The surface zone in caries and in caries-like lesions produced in vitro. Br Dent J. 1968. 125:145–157.38. Silverstone LM. The structure of carious enamel including the early lesions. Oral Sci Rev. 1973. 3:100–160.39. Silverstone LM. The significance of remineralization in caries prevention. J Can Dent Assoc. 1984. 50:157–167.40. Silverstone LM, Hicks MJ, Featherstone MJ. Dynamic factors affecting lesion initiation and progression in human dental enamel. 1. The dynamic nature of enamel caries. Quintessence Int. 1988. 19:683–711.41. Silverstone LM, Wefel JS, Zimmerman BF, Clarkson BH, Featherstone MJ. Reminaralization phenomena. Caries Res. 1977. 11:59–84.42. Silverstone LM, Wefel JS, Zimmerman BF, Clarkson BH, Featherstone MJ. Reminaralization of Natural and Artificial Lesions in Human Dental Enamel in vitro. Effect of calcium concentration of the calcifying fluid. Caries Res. 1981. 15:138–157.
Article43. Takaaki Y. Crystalline structure of enamel in carious lesion. J Jpn Dent Ass. 1997. 46:1167–1176.44. Yanagisawa T, Miake Y. High-resolution electron microscopy of enamel-crystal demineralization and remineralization in carious lesions. J Electron Microsc (Tokyo). 2003. 52:605–613.
Article45. Takuma S, Ogiwara H, Suzuki H. Electron-probe and electron microscope studies of carious dentinal lesions with a remineralized surface layer. Caries Res. 1975. 9(4):278–285.
Article46. Ten Cate JM. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982. 16:201–210.
Article47. Ten Cate JM. Review on fluoride, with special emphasis on calcium fluoride mechanism in caries prevention. Eur J Oral Sci. 1997. 105:461–465.
Article48. Tohda H, Yangisawa T, Takana N, Takuma S. Growth and fusion of apatite crystals in the remineralized enamel. J Electron Microsc (Tokyo). 1990. 39:238–244.49. Varughese K, Moreno EC. Crystal growth of calcium apatite in dilute solutions containing fluoride. Calcif Tissue Int. 1981. 33:431–439.
Article50. Warshawsky H, Nancy A. Stereo electron microscopy of enamel crystallites. J Dent Res. 1982. 61:1504–1514.51. Kum K, Lee C. The Effect of Four Kinds of Acid and Concentration on the Formation of Artificial Carious Lesion in Human Tooth Enamel. J Korean Acad Conserv Dent. 1996. 21:470–488.52. Kim M, Kum K, Lee C. The Influence of PH on Remineralization of Artificial Dental Caries. J Korean Acad Conserv Dent. 1997. 22:193–208.53. Kim SC. The Effect of The PH of Remineralized Buffer Solutions on Dentin Remineralizxation. 2005. Seoul: Yonsei University;Doctor's thesis.54. Park SH, Lee CY, Lee CS. The Effect of Acid Concentration and PH of Lactate Buffer Solution on the Progress of Artificial Caries Lesion in Human Tooth Enamel. J Korean Acad Conserv Dent. 1993. 18:277–290.55. Park J, Hur B, Lee C. The Effects of The Degree of Saturation of Acidulated Buffer Solutions in Enamel and Dentin Remineralization and AFM Observation of Hydroxyapatite Crystals. J Korean Acad Conserv Dent. 2000. 25:459–473.56. Oh H, Lee C. The Influence of PH and Lactic Acid Concentration on the Formation of Artificial Root Caries in Acid Buffer Solution. 2005. Seoul: Yonsei University;Doctor's thesis.57. Lee CY. Artificial Caries Formation in Acid Buffering Solution. Yonsei Dent J. 1992. 7:34–41.58. Han W, Kum K, Lee C. The Influence of Fluoride on Remineralization of Artificial Dental Caries. J Korean Acad Conserv Dent. 1996. 21:161–173.59. Han W, Lee C. The Effects of the Fluoride Concentration of Acidulated Buffer Solutions on Dentine Remineralization. 2004. Seoul: Yonsei University;Doctor's thesis.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The influence of pH and lactic acid concentration on the formation of artificial root caries in acid buffer solution
- The dynamic change of arttificially demineralized enamel by degree of saturation of remineralization at pH 4.3
- The remineralization aspect of enamel according to change of the degree of saturation of the organic acid buffering solution in pH 5.5
- The remineralizing features of pH 5.5 solutions of different degree of saturations on artificially demineralized enamel
- The effect of lactic acid concentration and ph of lactic acid buffer solutions on enamel remineralization