J Korean Soc Radiol.
2018 Aug;79(2):68-76. 10.3348/jksr.2018.79.2.68.
Detection of Recurrent/Residual Hepatocellular Carcinoma: Single-Center Retrospective Comparative Study Between Parenchymal Blood Volume Mapping Using Cone Beam CT and Multiphase Dynamic CT
- Affiliations
-
- 1Department of Radiology, Eulji University Hospital, Eulji University, Daejeon, Korea.
- 2Department of Radiology, Anyang Sam Hospital, Anyang, Korea. brtotips@gmail.com
- KMID: 2416391
- DOI: http://doi.org/10.3348/jksr.2018.79.2.68
Abstract
- PURPOSE
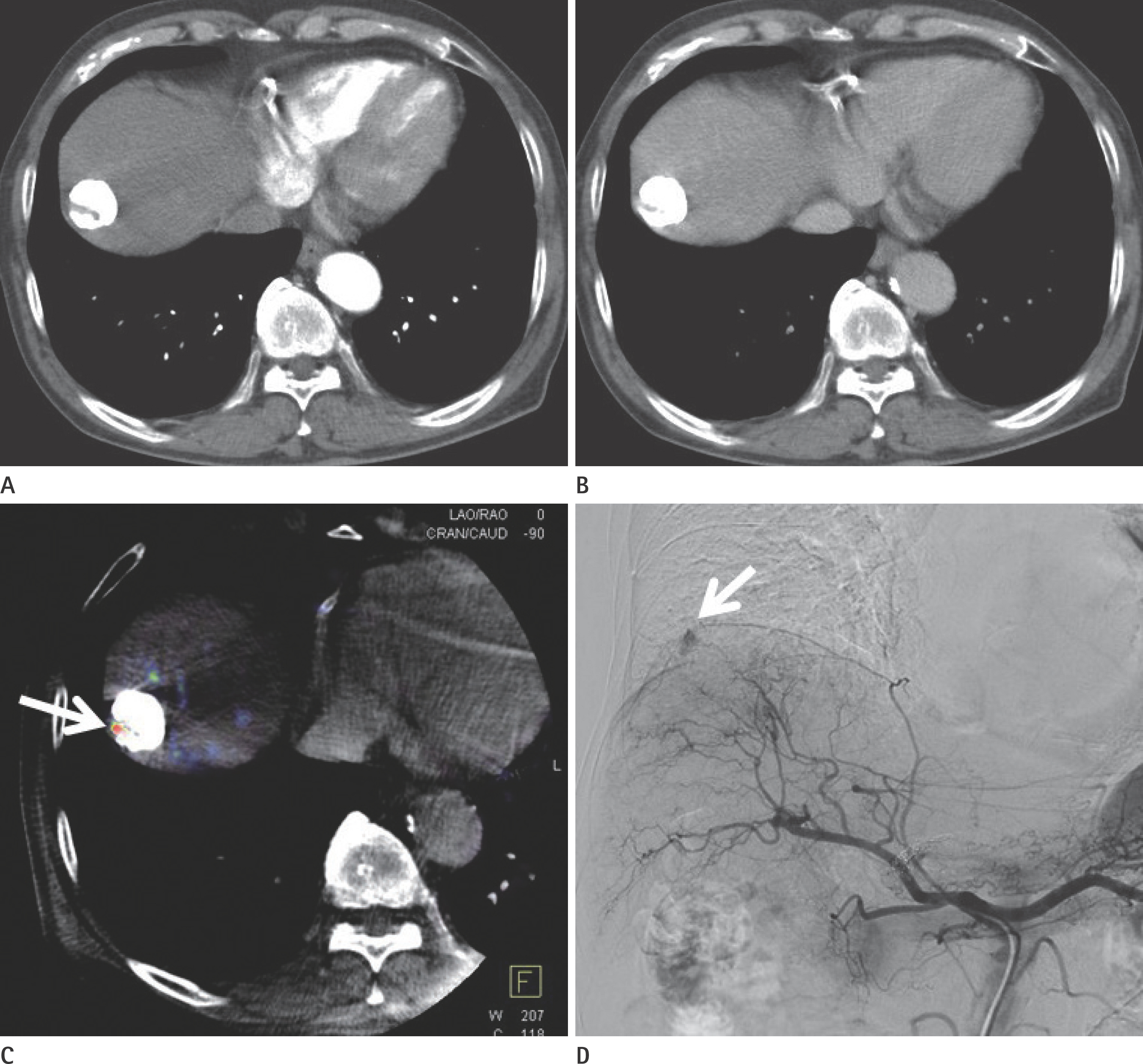
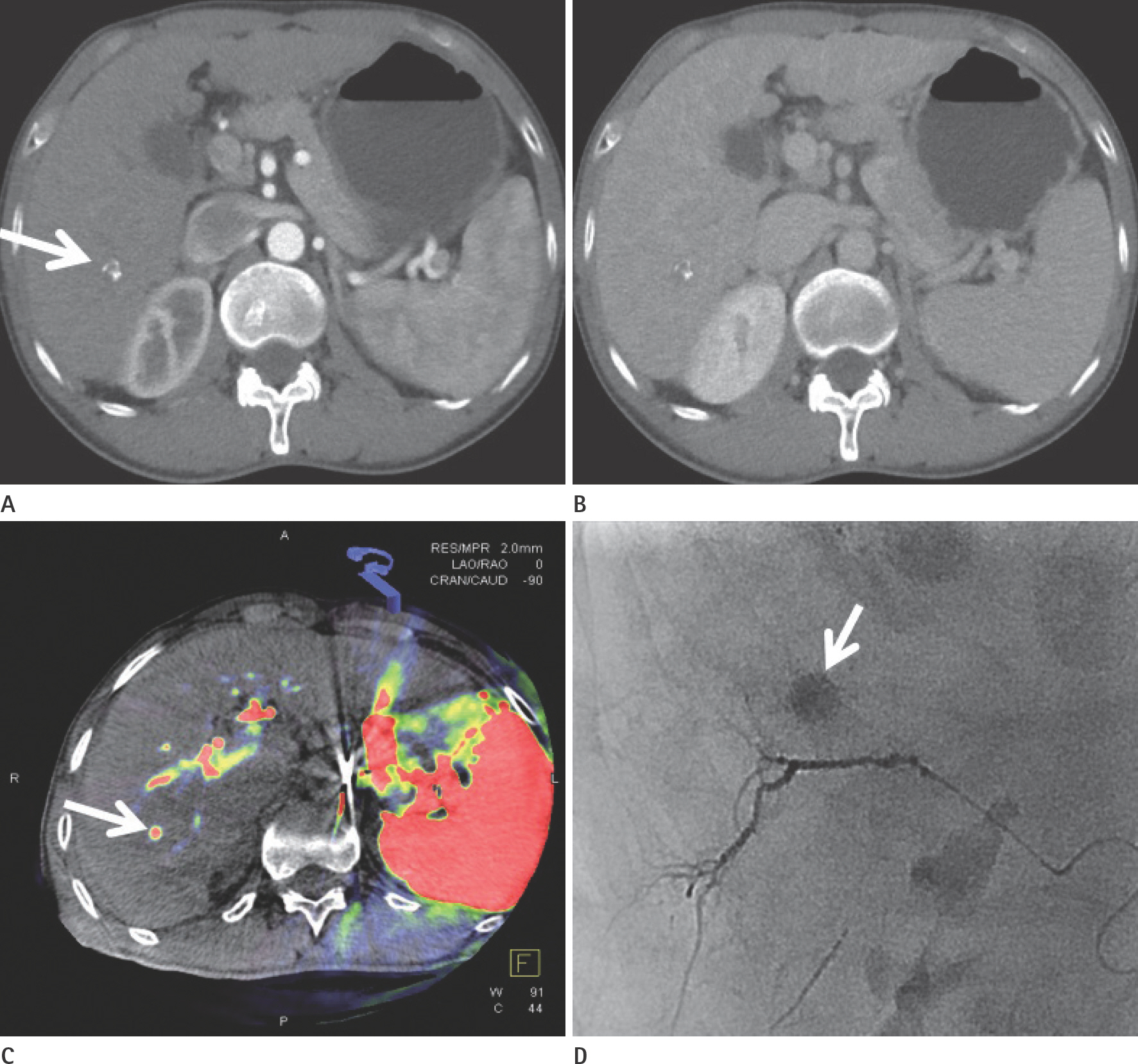
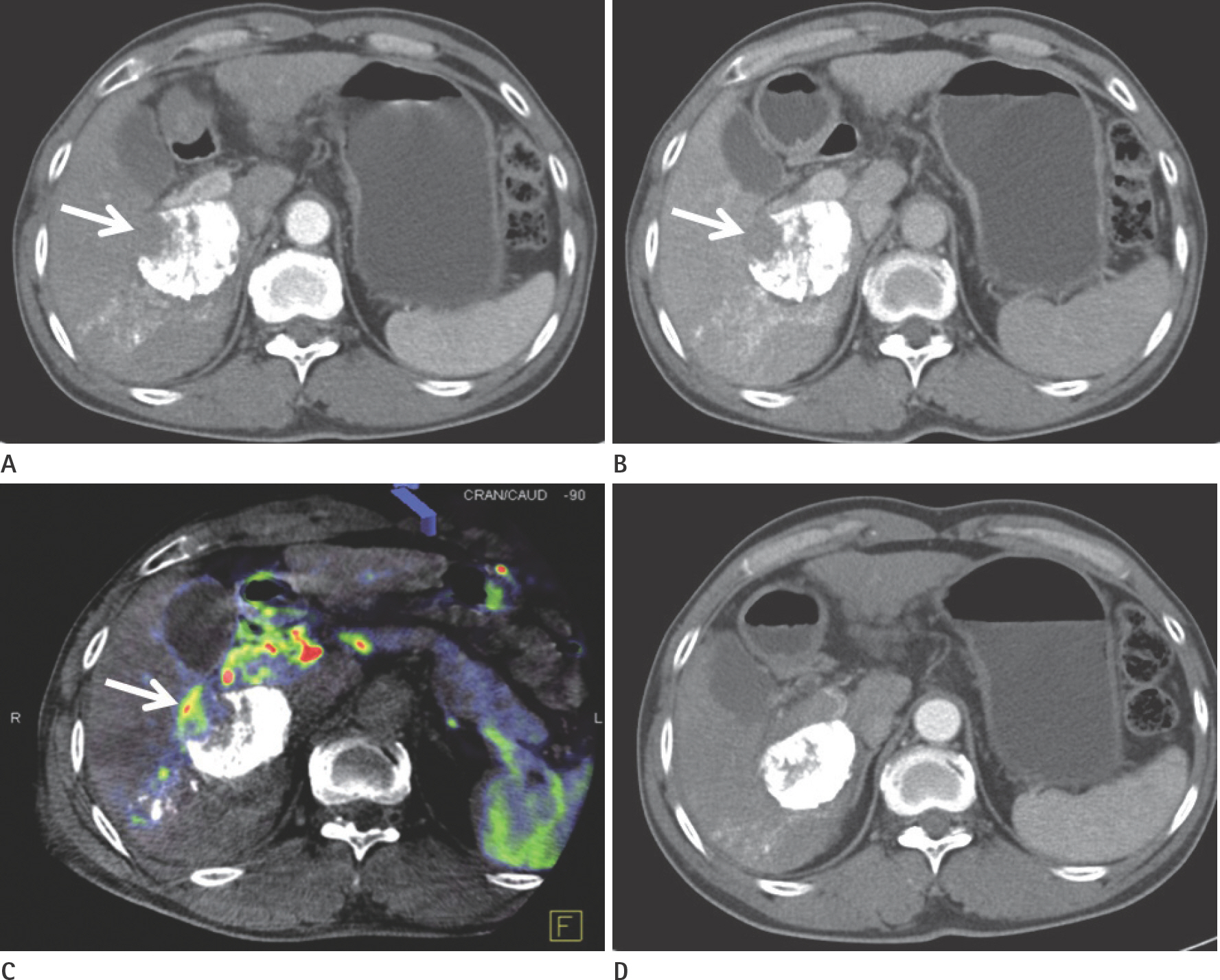
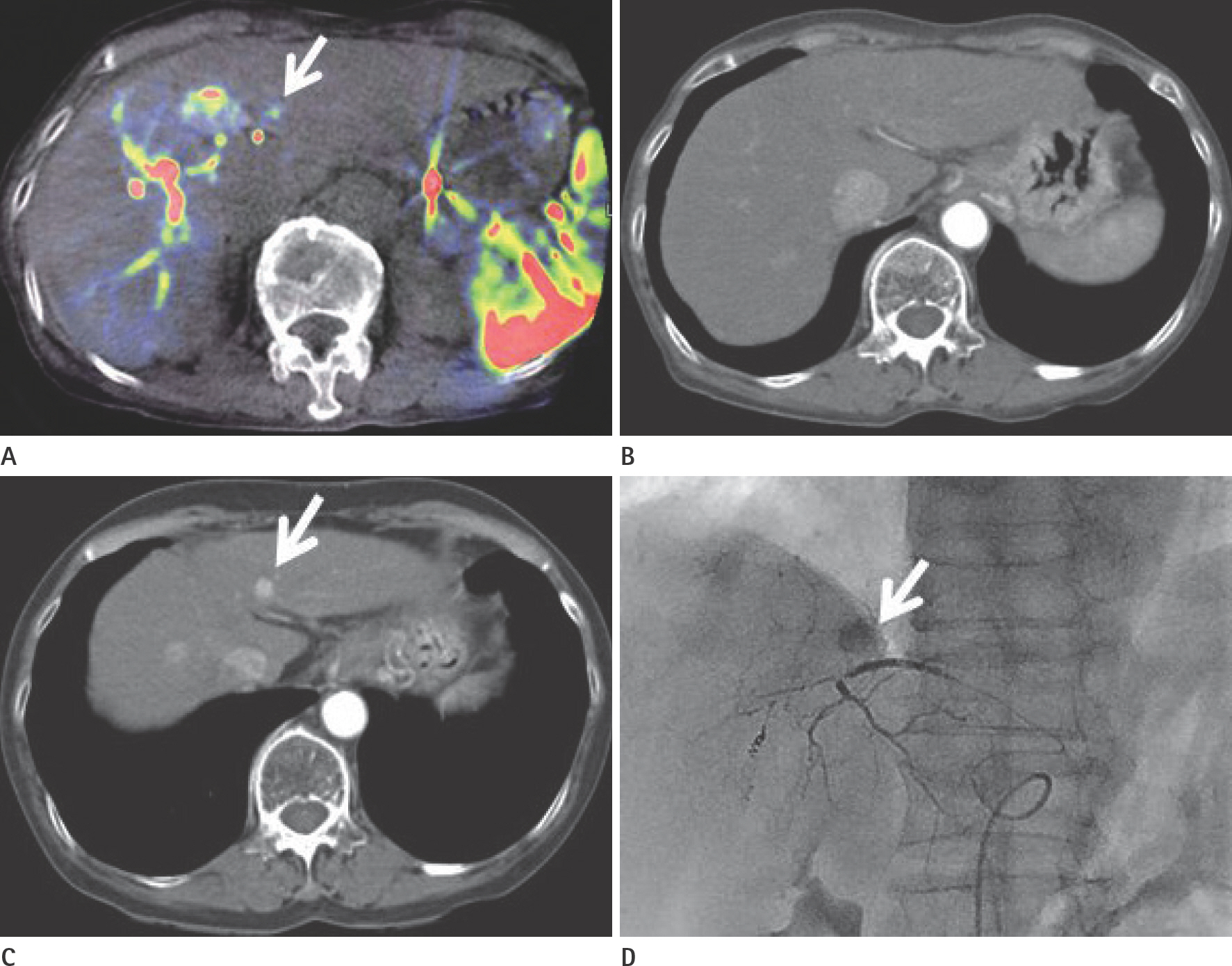
To evaluate the usefulness of cone beam computed tomography (CT)-based parenchymal blood volume (PBV) mapping for the detection of marginal recurrence or residual hepatocellular carcinoma, after transcatheter arterial chemoembolization (TACE), and to compare it with multiphase dynamic CT (MDCT).
MATERIALS AND METHODS
From March 2015 to October 2016, 26 patients with 49 iodized nodules who underwent TACE and a pre-interventional MDCT scan were enrolled in our study. We evaluated the diagnostic efficacies of PBV mapping using cone beam CT and MDCT in the detection of marginal recurrences or viable tumors.
RESULTS
The sensitivity, specificity, positive predictive value, and negative predictive value (NPV) of PBV mapping and MDCT were 100%, 96.7%, 94.7%, and 100%, and 77.9%, 93.5%, 87.5%, and 87.8%, respectively. The overall sensitivity for identifying local marginal recurrence was higher for PBV mapping than for MDCT (p < 0.005). The performances of PBV mapping and MDCT in the diagnosis of local marginal recurrence were significantly different (p = 0.037, McNemar test).
CONCLUSION
Compared with MDCT, PBV mapping can significantly increase the detection of marginal recurrence or residual tumor after TACE because it is free of beam-hardening artifact. PBV mapping should be considered as a feasible modality-related tool for patients who have undergone chemoembolization.
MeSH Terms
Figure
Reference
-
1.Vogl TJ., Schaefer P., Lehnert T., Nour-Eldin NE., Ackermann H., Mbalisike E, et al. Intraprocedural blood volume measurement using C-arm CT as a predictor for treatment response of malignant liver tumours undergoing repetitive transarterial chemoembolization (TACE). Eur Radiol. 2016. 26:755–763.
Article2.Yu JS. Hepatocellular carcinoma after transcatheter arterial chemoembolization: difficulties on imaging follow-up. Korean J Radiol. 2005. 6:134–135.
Article3.Kim SH., Lee WJ., Lim HK., Lim JH. Prediction of viable tumor in hepatocellular carcinoma treated with transcatheter arterial chemoembolization: usefulness of attenuation value measurement at quadruple-phase helical computed tomography. J Comput Assist Tomogr. 2007. 31:198–203.4.Choi SJ., Kim J., Seo J., Kim HS., Lee JM., Park H. Parametric response mapping of dynamic CT for predicting intrahepatic recurrence of hepatocellular carcinoma after conventional transcatheter arterial chemoembolization. Eur Radiol. 2016. 26:225–234.
Article5.Choi SH., Chung JW., Kim HC., Baek JH., Park CM., Jun S, et al. The role of perfusion CT as a follow-up modality after transcatheter arterial chemoembolization: an experimental study in a rabbit model. Invest Radiol. 2010. 45:427–436.6.Lee JK., Chung YH., Song BC., Shin JW., Choi WB., Yang SH, et al. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2002. 17:52–58.7.Kubota K., Hisa N., Nishikawa T., Fujiwara Y., Murata Y., Itoh S, et al. Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom Imaging. 2001. 26:184–190.
Article8.Choi JW., Kim HC., Lee JH., Yu SJ., Cho EJ., Kim MU, et al. Cone beam CT-guided chemoembolization of probable hepatocellular carcinomas smaller than 1 cm in patients at high risk of hepatocellular carcinoma. J Vasc Interv Radiol. 2017. 28:795–803. .e1.
Article9.Hunt SJ., Yu W., Weintraub J., Prince MR., Kothary N. Radiologic monitoring of hepatocellular carcinoma tumor viability after transhepatic arterial chemoembolization: estimating the accuracy of contrast-enhanced cross-sectional imaging with histopathologic correlation. J Vasc Interv Radiol. 2009. 20:30–38.
Article10.Kim HC. Role of C-arm cone-beam CT in chemoembolization for hepatocellular carcinoma. Korean J Radiol. 2015. 16:114–124.
Article11.Lee IJ., Chung JW., Yin YH., Kim HC., Kim YI., Jae HJ, et al. Conebeam CT hepatic arteriography in chemoembolization for hepatocellular carcinoma: angiographic image quality and its determining factors. J Vasc Interv Radiol. 2014. 25:1369–1379. quiz 79-e1.
Article12.Ahmed AS., Zellerhoff M., Strother CM., Pulfer KA., Redel T., Deuerling-Zheng Y, et al. C-arm CT measurement of cerebral blood volume: an experimental study in canines. AJNR Am J Neuroradiol. 2009. 30:917–922.
Article13.Kaufmann S., Horger T., Oelker A., Kloth C., Nikolaou K., Schulze M, et al. Characterization of hepatocellular carcinoma (HCC) lesions using a novel CT-based volume perfusion (VPCT) technique. Eur J Radiol. 2015. 84:1029–1035.
Article14.Bayraktutan Ü., Kantarci A., Og˘ul H., Kizrak Y., Özyig Ö., Yücel-er Z, et al. Evaluation of hepatocellular carcinoma with computed tomography perfusion imaging. Turk J Med Sci. 2014. 44:193–196.
Article15.Sahani DV., Holalkere NS., Mueller PR., Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue—initial experience. Radiology. 2007. 243:736–743.16.Petralia G., Fazio N., Bonello L., D'Andrea G., Radice D., Bellomi M. Perfusion computed tomography in patients with hepatocellular carcinoma treated with thalidomide: initial experience. J Comput Assist Tomogr. 2011. 35:195–201.17.Jiang T., Kambadakone A., Kulkarni NM., Zhu AX., Sahani DV. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST). Invest Radiol. 2012. 47:11–17.
Article18.Zhuang ZG., Zhang XB., Han JF., Beilner J., Deuerling-Zheng Y., Chi JC, et al. Hepatic blood volume imaging with the use of flat-detector CT perfusion in the angiography suite: comparison with results of conventional multislice CT perfusion. J Vasc Interv Radiol. 2014. 25:739–746.
Article19.Pung L., Ahmad M., Mueller K., Rosenberg J., Stave C., Hwang GL, et al. The role of cone-beam CT in transcatheter arterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Vasc Interv Radiol. 2017. 28:334–341.
Article20.Lucatelli P., Argirò R., Ginanni Corradini S., Saba L., Cirelli C., Fanelli F, et al. Comparison of image quality and diagnostic performance of cone-beam CT during drug-eluting embolic transarterial chemoembolization and multidetector CT in the detection of hepatocellular carcinoma. J Vasc Interv Radiol. 2017. 28:978–986.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of C-Arm Cone-Beam CT in Chemoembolization for Hepatocellular Carcinoma
- Three-dimensional imaging modalities in endodontics
- Detection of Recurrent Hepatocellular Carcinoma in Cirrhotic Liver after Transcatheter Arterial Chemoembolization: Value of Quantitative Color Mapping of the Arterial Enhancement Fraction of the Liver
- CT Detection of Hepatocellular Carcinoma in Advanced Liver Cirrhosis: Correlation of Helical CT and Explanted Liver
- Dynamic CT Finding of Pelioid HCC; Case Report