Yeungnam Univ J Med.
2018 Jun;35(1):76-83. 10.12701/yujm.2018.35.1.76.
Chemotherapy adherence is a favorable prognostic factor for elderly patients with multiple myeloma who are treated with a frontline bortezomib-containing regimen
- Affiliations
-
- 1Department of Hematology/Oncology, Kyungpook National University Hospital, Daegu, Korea. jhmoon@knu.ac.kr
- 2Department of Hematology/Oncology, Kosin University Gospel Hospital, Busan, Korea.
- 3Department of Hematology-Oncology, Inje University Pusan Baik Hospital, Busan, Korea.
- 4Department of Hematology-Oncology, Dong-A University Hospital, Busan, Korea.
- KMID: 2415737
- DOI: http://doi.org/10.12701/yujm.2018.35.1.76
Abstract
- BACKGROUND
Elderly patients with multiple myeloma (MM) are vulnerable to adverse events (AEs). This study evaluated adherence to chemotherapy and treatment outcomes in elderly patients treated with a frontline bortezomib (BTZ), melphalan, and prednisone (VMP) regimen and regimens without BTZ.
METHODS
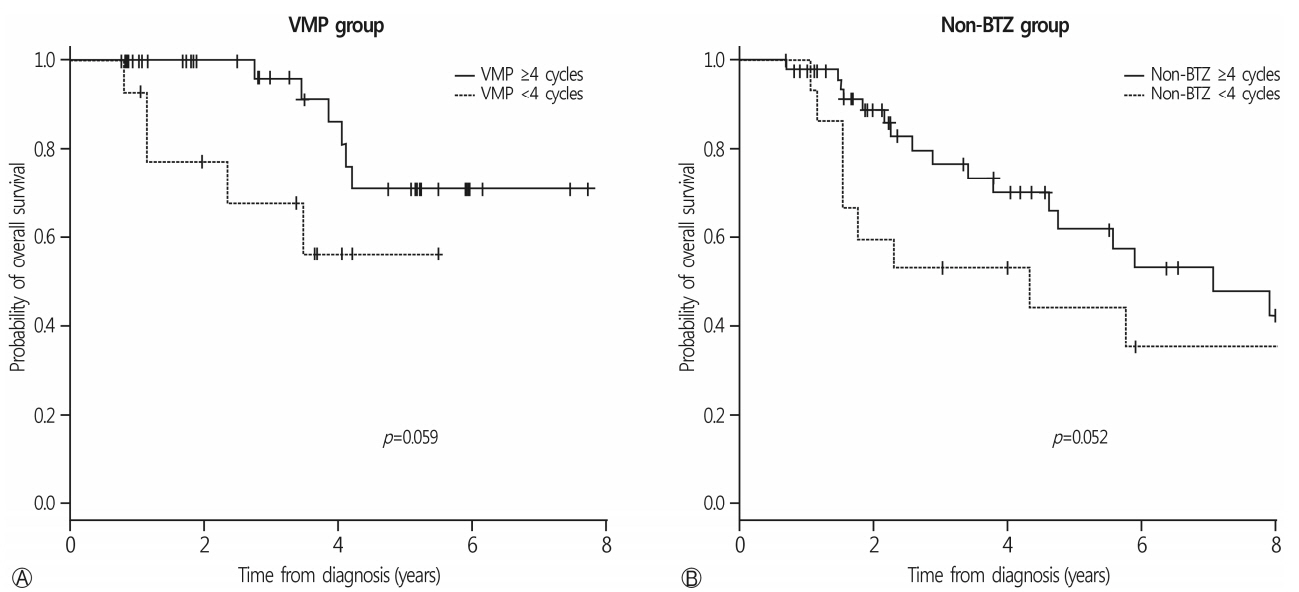
One-hundred and forty elderly patients who were diagnosed with MM from March 2007 to March 2015 were included in this retrospective study. To evaluate regimen adherence, patients who were treated with more than 4 cycles were assigned to the good adherence group.
RESULTS
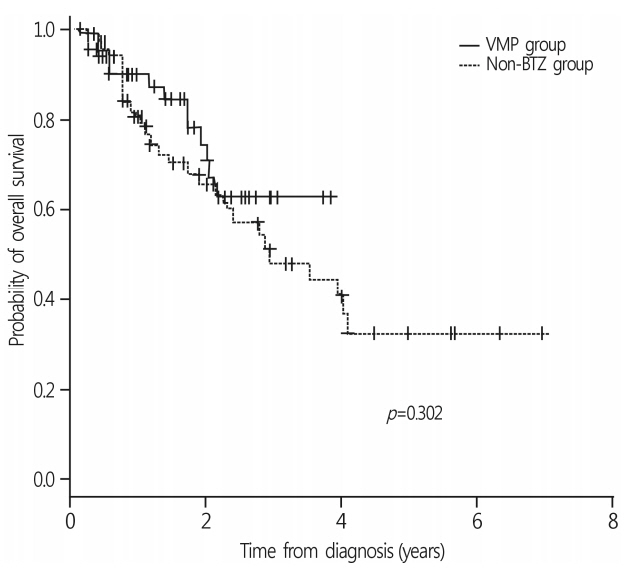
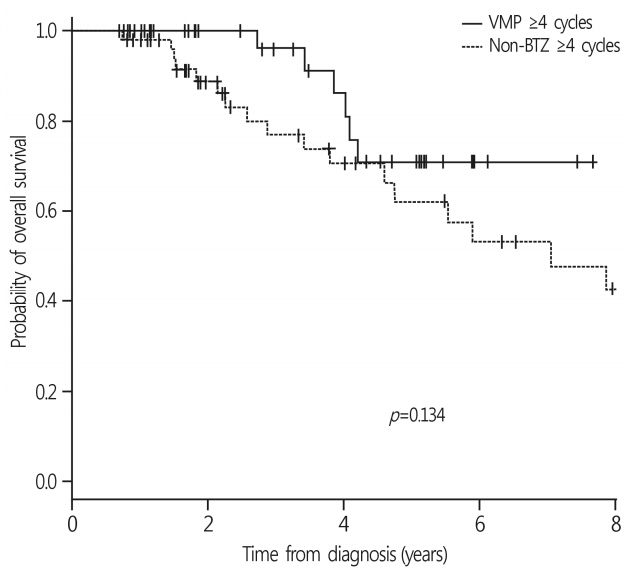
Among the 140 patients, 71 were treated with a frontline VMP and 69 with non-BTZ regimens. The median age was 71 years (range, 65-90 years). The VMP group showed a higher complete response rate than the non-BTZ group: 26.8% vs. 7.2%. More patients in the VMP group achieved ≥ very good partial response (VGPR) and ≥ PR. In the VMP group, 27 patients (38.0%) received less than 4 cycles. The VMP good adherence group showed a higher 3-year overall survival (OS) rate (70.9%) than the poor adherence group (60.2%, p=0.059). In the multivariate analysis, treatment with ≥ 4 cycles of VMP was a favorable factor for OS.
CONCLUSION
A good adherence to a frontline VMP regimen resulted in favorable long-term survival. Adequate management of AEs will be needed to achieve favorable outcomes in elderly patients with MM.
Keyword
MeSH Terms
Figure
Reference
-
1. Gregory WM, Richards MA, Malpas JS. Combination chemotherapy versus melphalan and prednisolone in the treatment of multiple myeloma: an overview of published trials. J Clin Oncol. 1992; 10:334–42.
Article2. Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006; 367:825–31.
Article3. Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008; 112:3107–14.
Article4. San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008; 359:906–17.
Article5. Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012; 366:1759–69.
Article6. San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol. 2013; 31:448–55.
Article7. Palumbo A, Bringhen S, Ludwig H, Dimopoulos MA, Bladé J, Mateos MV, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011; 118:4519–29.
Article8. Gay F, Palumbo A. Management of older patients with multiple myeloma. Blood Rev. 2011; 25:65–73.
Article9. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014; 15:e538–48.
Article10. Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011; 12:431–40.
Article11. Yang DH, Kim YK, Sohn SK, Chung JS, Joo YD, Lee JH, et al. Induction treatment with cyclophosphamide, thalidomide, and dexamethasone in newly diagnosed multiple myeloma: a phase II study. Clin Lymphoma Myeloma Leuk. 2010; 10:62–7.
Article12. Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006; 20:1467–73.
Article13. Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014; 106:pii: dju244.14. Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983; 1:710–9.
Article15. Harousseau JL, Palumbo A, Richardson PG, Schlag R, Dimopoulos MA, Shpilberg O, et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: analysis of the phase 3 VISTA study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood. 2010; 116:3743–50.
Article16. Delforge M, Dhawan R, Robinson D Jr, Meunier J, Regnault A, Esseltine DL, et al. Health-related quality of life in elderly, newly diagnosed multiple myeloma patients treated with VMP vs. MP: results from the VISTA trial. Eur J Haematol. 2012; 89:16–27.
Article17. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6, 633 patients from 27 randomized trials. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. Myeloma Trialists' Collaborative Group. J Clin Oncol. 1998; 16:3832–42.
Article18. Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008; 111:2516–20.
Article19. Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014; 28:1122–8.
Article20. Pozzi S, Marcheselli L, Bari A, Liardo EV, Marcheselli R, Luminari S, et al. Survival of multiple myeloma patients in the era of novel therapies confirms the improvement in patients younger than 75 years: a population-based analysis. Br J Haematol. 2013; 163:40–6.
Article21. Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010; 28:2259–66.
Article22. Vestal RE. Aging and pharmacology. Cancer. 1997; 80:1302–10.
Article23. Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997; 61:331–9.
Article24. Palumbo A, Gay F, Cavallo F, Di Raimondo F, Larocca A, Hardan I, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J Clin Oncol. 2015; 33:3459–66.
Article25. Larocca A, Palumbo A. How I treat fragile myeloma patients. Blood. 2015; 126:2179–85.
Article26. Delforge M, Minuk L, Eisenmann JC, Arnulf B, Canepa L, Fragasso A, et al. Health-related quality-of-life in patients with newly diagnosed multiple myeloma in the FIRST trial: lenalidomide plus low-dose dexamethasone versus melphalan, prednisone, thalidomide. Haematologica. 2015; 100:826–33.
Article27. van der Poel MW, Oerlemans S, Schouten HC, van de Poll-Franse LV. Elderly multiple myeloma patients experience less deterioration in health-related quality of life than younger patients compared to a normative population: a study from the population-based PROFILES registry. Ann Hematol. 2015; 94:651–61.
Article28. Jung SH, Ahn JS, Yang DH, Cho MS, Kim JY, Ahn SY, et al. Oliguria as an early indicator of mortality risk in patients with multiple myeloma and renal impairment. Blood Res. 2015; 50:167–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reversible Heart Failure after Bortezomib Treatment in a Patient with Multiple Myeloma
- Poor prognostic significance of Mycobacterium tuberculosis infection during bortezomib-containing chemotherapy in patients with multiple myeloma
- A Case of Acute Pancreatitis Caused by Bortezomib in a Patient with Multiple Myeloma
- A Case of Cutaneous Plasmacytoma Treated with Bortezomib and Radiotherapy
- Huge Cutaneous Involvement of Multiple Myeloma