Characteristics of Synchronous and Metachronous Multiple Gastric Tumors after Endoscopic Submucosal Dissection of Early Gastric Neoplasm
- Affiliations
-
- 1Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea. chokb@dsmc.or.kr
- 2Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2414868
- DOI: http://doi.org/10.5946/ce.2017.109
Abstract
- BACKGROUND/AIMS
Endoscopic submucosal dissection (ESD) has been widely accepted as a method of treatment of early gastric tumor. This study aimed to identify the incidence and characteristics of multiple gastric tumors after ESD.
METHODS
Patients with early gastric tumors who were treated by ESD from January 2004 to June 2012 and followed up with endoscopic examination periodically for at least 1 year were enrolled. All multiple gastric lesions were subsequently treated with ESD and the medical records of the patients were retrospectively reviewed.
RESULTS
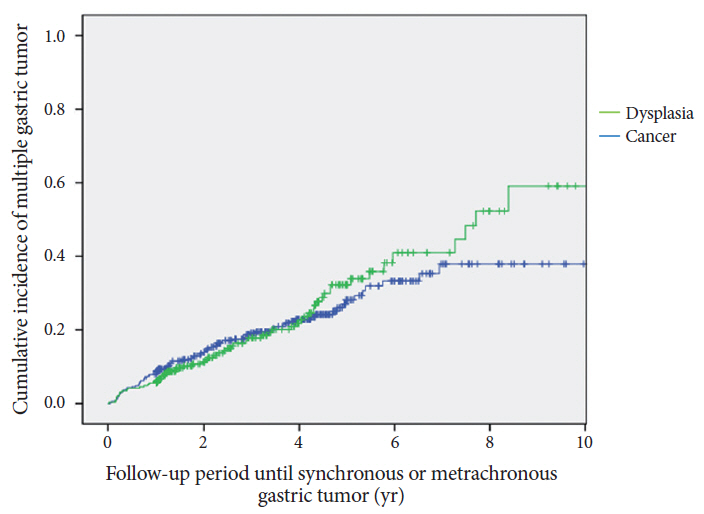
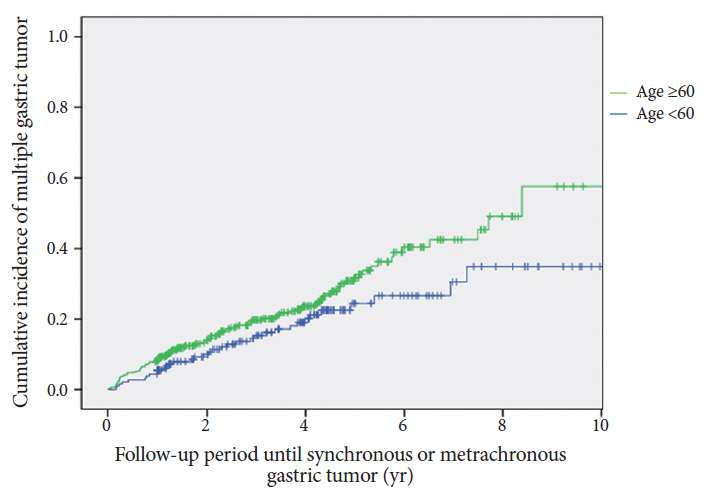
In total, 643 patients were included. The mean duration of endoscopic follow-up was 45.27±27.59 (range, 12-148) months. Overall, 144 patients (22.4%) showed multiple gastric tumors during the follow-up period (44 synchronous [6.8%] and 100 metachronous [15.5%]). The cumulative incidence rate steadily increased during the follow-up period. More than 50% of the tumors that developed at the same longitudinal location of the stomach were of the same macroscopic and histological type as the primary lesions.
CONCLUSIONS
Because synchronous and/or metachronous gastric tumors are common, considerable attention should be paid to detect multiple gastric lesions after ESD of early gastric neoplasm.
MeSH Terms
Figure
Cited by 6 articles
-
Common Locations of Gastric Cancer: Review of Research from the Endoscopic Submucosal Dissection Era
Su Jin Kim, Cheol Woong Choi
J Korean Med Sci. 2019;34(35):. doi: 10.3346/jkms.2019.34.e231.Editors' Choice of Noteworthy Clinical Endoscopy Publications in the First Decade
Gwang Ha Kim, Kwang An Kwon, Do Hyun Park, Jimin Han
Clin Endosc. 2021;54(5):633-640. doi: 10.5946/ce.2021.216.Effectiveness of Autologous Platelet-Rich Plasma for the Healing of Ulcers after Endoscopic Submucosal Dissection
Eunju Jeong, In kyung Yoo, Ozlem Ozer Cakir, Hee Kyung Kim, Won Hee Kim, Sung Pyo Hong, Joo Young Cho
Clin Endosc. 2019;52(5):472-478. doi: 10.5946/ce.2018.152.Immunohistochemical Expression of Epithelial-Mesenchymal Transition Markers in Early Gastric Cancer: Cancer Tissue versus Noncancer Tissue
Hee Jae Jung, Su Jin Hong, Shin Hee Kim
Clin Endosc. 2019;52(5):464-471. doi: 10.5946/ce.2018.181.Assessment of Endoscopic Gastric Atrophy according to the Kimura-Takemoto Classification and Its Potential Application in Daily Practice
Duc Trong Quach, Toru Hiyama
Clin Endosc. 2019;52(4):321-327. doi: 10.5946/ce.2019.072.Clinical Implications of Synchronous and Metachronous Multiple Gastric Tumors after Endoscopic Resection of Gastric Neoplasms
Cheol Min Shin
Clin Endosc. 2018;51(3):209-210. doi: 10.5946/ce.2018.074.
Reference
-
1. Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981; 11:127–139.2. Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br J Surg. 1992; 79:241–244.
Article3. Lauwers GY, Riddell RH. Gastric epithelial dysplasia. Gut. 1999; 45:784–790.
Article4. de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007; 12:1–15.
Article5. Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD study group multicenter study. Gastrointest Endosc. 2009; 69:1228–1235.
Article6. Nakajima T, Oda I, Gotoda T, et al. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer. 2006; 9:93–98.
Article7. Ahn JY, Jung HY. Long-term outcome of extended endoscopic submucosal dissection for early gastric cancer with differentiated histology. Clin Endosc. 2013; 46:463–466.
Article8. Choi CW, Kang DH, Kim HW, Park SB, Kim S, Cho M. Endoscopic submucosal dissection as a treatment for gastric adenomatous polyps: predictive factors for early gastric cancer. Scand J Gastroenterol. 2012; 47:1218–1225.
Article9. Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005; 3(7 Suppl 1):S71–S73.
Article10. Kim YY, Jeon SW, Kim J, et al. Endoscopic submucosal dissection for early gastric cancer with undifferentiated histology: could we extend the criteria beyond? Surg Endosc. 2013; 27:4656–4662.
Article11. Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009; 58:331–336.
Article12. Yoo JH, Shin SJ, Lee KM, et al. How can we predict the presence of missed synchronous lesions after endoscopic submucosal dissection for early gastric cancers or gastric adenomas? J Clin Gastroenterol. 2013; 47:e17–e22.
Article13. Nitta T, Egashira Y, Akutagawa H, et al. Study of clinicopathological factors associated with the occurrence of synchronous multiple gastric carcinomas. Gastric Cancer. 2009; 12:23–30.
Article14. Lee HL, Eun CS, Lee OY, et al. When do we miss synchronous gastric neoplasms with endoscopy? Gastrointest Endosc. 2010; 71:1159–1165.
Article15. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma - 2nd English edition. Gastric Cancer. 1998; 1:10–24.16. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58(6 Suppl):S3–S43.17. Moertel CG, Bargen JA, Soule EH. Multiple gastric cancers; review of the literature and study of 42 cases. Gastroenterology. 1957; 32:1095–1103.18. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000; 47:251–255.
Article19. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.20. Hosokawa O, Kaizaki Y, Watanabe K, et al. Endoscopic surveillance for gastric remnant cancer after early cancer surgery. Endoscopy. 2002; 34:469–473.
Article21. Park CH, Lee H, Kim DW, et al. Clinical safety of endoscopic submucosal dissection compared with surgery in elderly patients with early gastric cancer: a propensity-matched analysis. Gastrointest Endosc. 2014; 80:599–609.22. Arima N, Adachi K, Katsube T, et al. Predictive factors for metachronous recurrence of early gastric cancer after endoscopic treatment. J Clin Gastroenterol. 1999; 29:44–47.
Article23. Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005; 37:990–993.
Article24. Han JS, Jang JS, Choi SR, et al. A study of metachronous cancer after endoscopic resection of early gastric cancer. Scand J Gastroenterol. 2011; 46:1099–1104.
Article25. Goddard AF, Badreldin R, Pritchard DM, Walker MM, Warren B; British Society of Gastroenterology. The management of gastric polyps. Gut. 2010; 59:1270–1276.
Article26. Kato M, Nishida T, Yamamoto K, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka university ESD study group. Gut. 2013; 62:1425–1432.
Article27. Ha TK, An JY, Youn HG, et al. Missed lesions in synchronous multiple gastric cancer. ANZ J Surg. 2010; 80:276–279.
Article28. Gong EJ, Lee JH, Jung K, et al. Characteristics of missed simultaneous gastric lesions based on double-check analysis of the endoscopic image. Clin Endosc. 2017; 50:261–269.
Article29. Kobayashi M, Narisawa R, Sato Y, Takeuchi M, Aoyagi Y. Self-limiting risk of metachronous gastric cancers after endoscopic resection. Dig Endosc. 2010; 22:169–173.
Article30. Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc. 2016; 84:628–638. e1.
Article31. Choi IJ. Endoscopic gastric cancer screening and surveillance in high-risk groups. Clin Endosc. 2014; 47:497–503.
Article32. Lee H, Yun WK, Min BH, et al. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2011; 25:1985–1993.
Article33. Cahill RJ, Kilgallen C, Beattie S, Hamilton H, O’Morain C. Gastric epithelial cell kinetics in the progression from normal mucosa to gastric carcinoma. Gut. 1996; 38:177–181.
Article34. Esaki Y, Hirokawa K, Yamashiro M. Multiple gastric cancers in the aged with special reference to intramucosal cancers. Cancer. 1987; 59:560–565.
Article35. Kato M, Asaka M, Ono S, et al. Eradication of Helicobacter pylori for primary gastric cancer and secondary gastric cancer after endoscopic mucosal resection. J Gastroenterol. 2007; 42 Suppl 17:16–20.
Article36. Seo JH, Park JC, Kim YJ, Shin SK, Lee YC, Lee SK. Undifferentiated histology after endoscopic resection may predict synchronous and metachronous occurrence of early gastric cancer. Digestion. 2010; 81:35–42.
Article37. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008; 372:392–397.
Article38. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--first American cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 1992; 52:6735–6740.39. Chon I, Choi C, Shin CM, Park YS, Kim N, Lee DH. Effect of Helicobacter pylori eradication on subsequent dysplasia development after endoscopic resection of gastric dysplasia. Korean J Gastroenterol. 2013; 61:307–312.40. Maehata Y, Nakamura S, Fujisawa K, et al. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012; 75:39–46.
Article41. Choi J, Kim SG, Yoon H, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2014; 12:793–800. e1.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinicopathological characteristics of synchronous and metachronous gastric neoplasms after endoscopic submucosal dissection
- A Case of Metachronous Gastric Cancer and Follicular Lymphoma after Endoscopic Submucosal Dissection for Early Gastric Cancer
- Pathological Interpretation of Gastric Tumors in Endoscopic Submucosal Dissection
- The pattern of metachronous recurrence after endoscopic submucosal dissection for gastric adenocarcinoma and dysplasias
- Risk Factors for Metachronous Recurrence after Endoscopic Submucosal Dissection of a Gastric Neoplasm