Investig Clin Urol.
2017 Nov;58(6):385-399. 10.4111/icu.2017.58.6.385.
Oxidation-reduction potential as a new marker for oxidative stress: Correlation to male infertility
- Affiliations
-
- 1American Center for Reproductive Medicine, Department of Urology, Cleveland Clinic, Cleveland, OH, USA. agarwaa@ccf.org
- 2Ohio University Heritage College of Osteopathic Medicine, Athens, OH, USA.
- KMID: 2414765
- DOI: http://doi.org/10.4111/icu.2017.58.6.385
Abstract
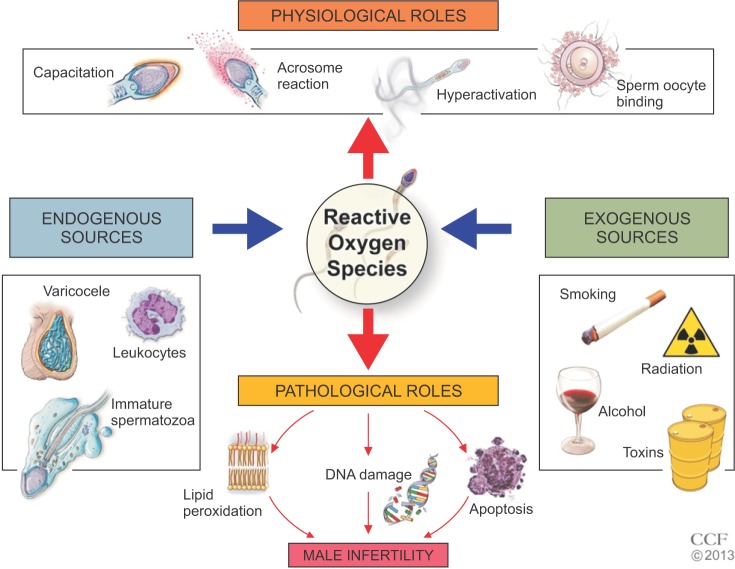
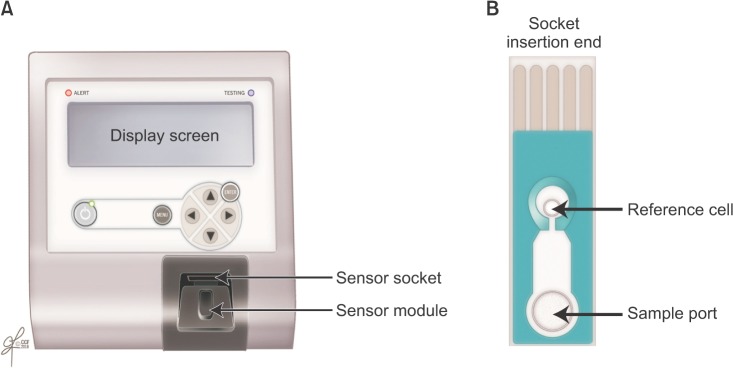
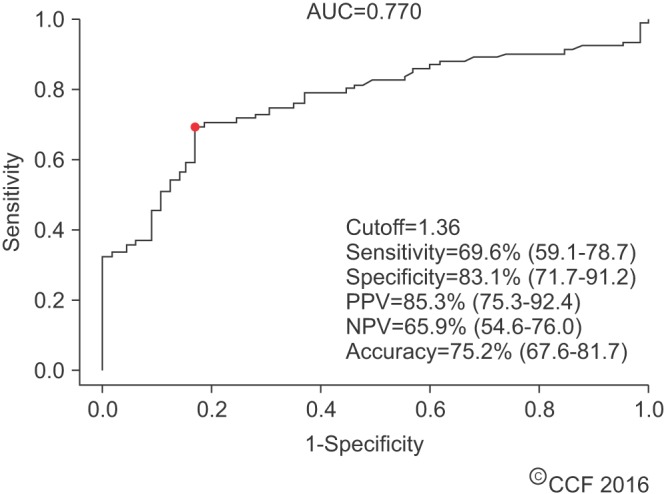
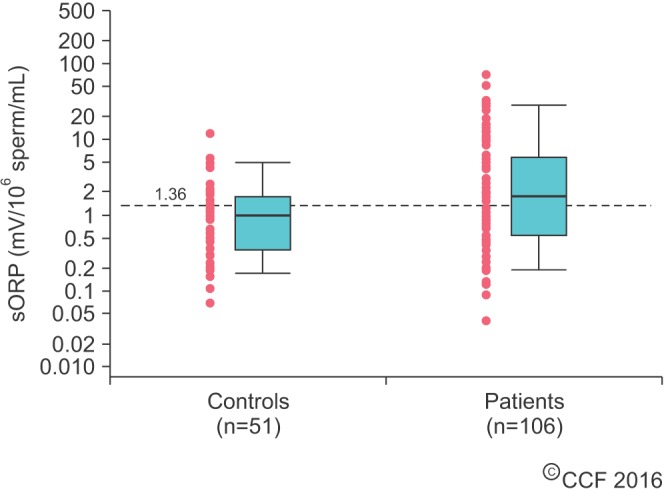
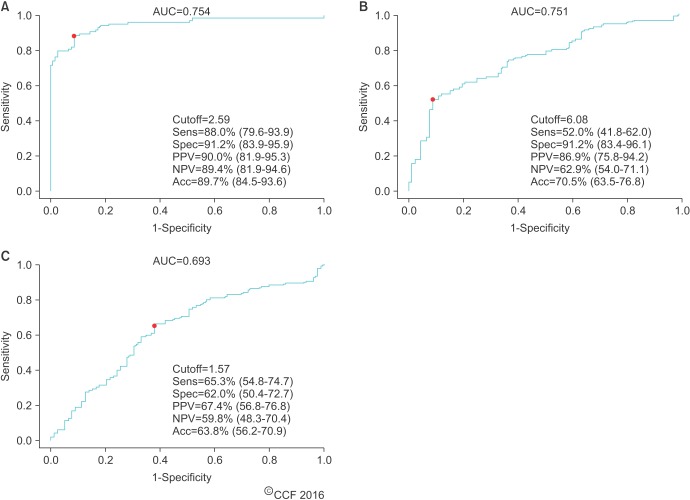
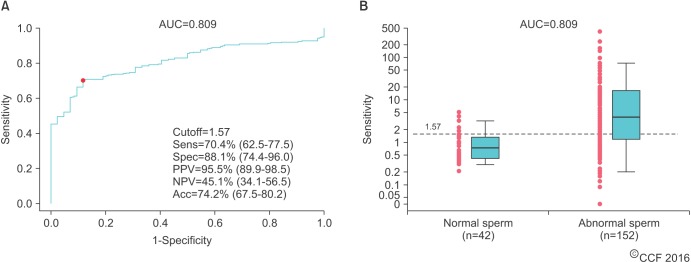
- Male infertility affects men worldwide. Oxidative stress (OS), characterized by an overabundance of reactive oxygen species (ROS) or a deficiency of antioxidants, is one of the major causes of male infertility. OS causes damage at the molecular level, which impairs lipids, proteins, and DNA. The cyclic cascade of redox reactions weakens sperm function which leads to poor semen parameters and eventual sterility. There is a need for advanced diagnostic tests that can quickly and accurately detect OS. Most commonly used assays can only measure single constituents of OS. However, the MiOXSYS System introduces a new strategy to detect OS by measuring the oxidation-reduction potential (ORP)--a direct evaluation of the redox balance between ROS and antioxidants. The MiOXSYS System has shown promise as a diagnostic tool in the evaluation of male infertility. This review explores the concept of ORP, details the principle of the MiOXSYS System, and summarizes the findings in clinical studies that support ORP measurement in semen.
MeSH Terms
Figure
Reference
-
1. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015; 8:191–196. PMID: 26752853.
Article2. Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014; 40:443–453. PMID: 25254609.
Article3. Esteves SC, Hamada A, Kondray V, Pitchika A, Agarwal A. What every gynecologist should know about male infertility: an update. Arch Gynecol Obstet. 2012; 286:217–229. PMID: 22392488.
Article4. Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES Jr, Agarwal A. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012; 79:16–22. PMID: 22070891.
Article5. Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987; 81:459–469. PMID: 2828610.
Article6. Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987; 8:338–348. PMID: 2822642.
Article7. Aitken RJ, Clarkson JS, Hargreave TB, Irvine DS, Wu FC. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J Androl. 1989; 10:214–220. PMID: 2501260.
Article8. Bisht S, Dada R. Oxidative stress: major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front Biosci (Schol Ed). 2017; 9:420–447. PMID: 28410127.9. de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod. 1997; 2:48–54. PMID: 9414465.
Article10. Du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. 2015; 32:509–520. PMID: 25646893.
Article11. Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017; 84:1039–1052. PMID: 28749007.
Article12. Imam SN, Shamsi MB, Kumar K, Deka D, Dada R. Idiopathic recurrent pregnancy loss: role of paternal factors; a pilot study. J Reprod Infertil. 2011; 12:267–276. PMID: 23926513.13. Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, Kammoun M, Meniaoui I, Sallem A, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril. 2016; 105:58–64. PMID: 26493117.
Article14. Henkel R, Kierspel E, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, et al. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod Biomed Online. 2003; 7:477–484. PMID: 14656411.
Article15. Du Plessis SS, Makker K, Desai NR, Agarwal A. Impact of oxidative stress on IVF. Expert Rev Obstet Gynecol. 2008; 3:539–554.
Article16. Yang Q, Zhao F, Hu L, Bai R, Zhang N, Yao G, et al. Effect of paternal overweight or obesity on IVF treatment outcomes and the possible mechanisms involved. Sci Rep. 2016; 6:29787. PMID: 27412918.
Article17. Opuwari CS, Henkel RR. An update on oxidative damage to spermatozoa and oocytes. Biomed Res Int. 2016; 2016:9540142. PMID: 26942204.
Article18. Agarwal A, Allamaneni SS, Said TM. Chemiluminescence technique for measuring reactive oxygen species. Reprod Biomed Online. 2004; 9:466–468. PMID: 15511350.
Article19. Said TM, Kattal N, Sharma RK, Sikka SC, Thomas AJ Jr, Mascha E, et al. Enhanced chemiluminescence assay vs colorimetric assay for measurement of the total antioxidant capacity of human seminal plasma. J Androl. 2003; 24:676–680. PMID: 12954657.
Article20. Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ Jr, Agarwal A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999; 14:2801–2807. PMID: 10548626.
Article21. Grotto D, Santa Maria LD, Boeira S, Valentini J, Charão MF, Moro AM, et al. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharm Biomed Anal. 2007; 43:619–624. PMID: 16949242.
Article22. Agarwal A, Roychoudhury S, Bjugstad KB, Cho CL. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol. 2016; 8:302–318. PMID: 27695529.
Article23. Shapiro HM. Redox balance in the body: an approach to quantitation. J Surg Res. 1972; 13:138–152. PMID: 4343247.
Article24. Bjugstad KB, Rael LT, Levy S, Carrick M, Mains CW, Slone DS, et al. Oxidation-reduction potential as a biomarker for severity and acute outcome in traumatic brain injury. Oxid Med Cell Longev. 2016; 2016:6974257. PMID: 27642494.
Article25. Bjugstad KB, Fanale C, Wagner J, Jensen J, Salottolo K, Rael LT, Bar-Or D. A 24 h delay in the redox response distinguishes the most severe stroke patients from less severe stroke patients. J Neurol Neurophys. 2016; 7:1000395.
Article26. Rael LT, Bar-Or R, Aumann RM, Slone DS, Mains CW, Bar-Or D. Oxidation-reduction potential and paraoxonase-arylesterase activity in trauma patients. Biochem Biophys Res Commun. 2007; 361:561–565. PMID: 17662690.
Article27. Rael LT, Bar-Or R, Mains CW, Slone DS, Levy AS, Bar-Or D. Plasma oxidation-reduction potential and protein oxidation in traumatic brain injury. J Neurotrauma. 2009; 26:1203–1211. PMID: 19317602.
Article28. Bobe G, Cobb TJ, Leonard SW, Aponso S, Bahro CB, Koley D, et al. Increased static and decreased capacity oxidation-reduction potentials in plasma are predictive of metabolic syndrome. Redox Biol. 2017; 12:121–128. PMID: 28222379.
Article29. Bar-Or R, Rael LT, Curtis CG, Mains CW, Slone DS, Bar-Or D. Raman spectral signatures of human liver perfusates correlate with oxidation reduction potential. Mol Med Rep. 2009; 2:175–180. PMID: 21475809.
Article30. Spanidis Y, Goutzourelas N, Stagos D, Kolyva AS, Gogos CA, Bar-Or D, et al. Assessment of oxidative stress in septic and obese patients using markers of oxidation-reduction potential. In Vivo. 2015; 29:595–600. PMID: 26359419.31. Stagos D, Goutzourelas N, Bar-Or D, Ntontou AM, Bella E, Becker AT, et al. Application of a new oxidation-reduction potential assessment method in strenuous exercise-induced oxidative stress. Redox Rep. 2015; 20:154–162. PMID: 25494543.
Article32. Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2016; 28:1–10. PMID: 27062870.
Article33. Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed (Yazd). 2016; 14:231–240. PMID: 27351024.
Article34. de Lamirande E, Leduc BE, Iwasaki A, Hassouna M, Gagnon C. Increased reactive oxygen species formation in semen of patients with spinal cord injury. Fertil Steril. 1995; 63:637–642. PMID: 7851599.35. Aprioku JS. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil. 2013; 14:158–172. PMID: 24551570.36. Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003; 79:829–843. PMID: 12749418.
Article37. Łuczaj W, Skrzydlewska E. DNA damage caused by lipid peroxidation products. Cell Mol Biol Lett. 2003; 8:391–413. PMID: 12813574.38. Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, Baker MA. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem. 2012; 287:33048–33060. PMID: 22851170.
Article39. Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012; 2012:646354. PMID: 21977319.
Article40. Agarwal A, Allamaneni SS, Nallella KP, George AT, Mascha E. Correlation of reactive oxygen species levels with the fertilization rate after in vitro fertilization: a qualified meta-analysis. Fertil Steril. 2005; 84:228–231. PMID: 16009190.
Article41. Agarwal A, Prabakaran S, Allamaneni SS. Relationship between oxidative stress, varicocele and infertility: a meta-analysis. Reprod Biomed Online. 2006; 12:630–633. PMID: 16790111.
Article42. Condorelli RA, Russo GI, Calogero AE, Morgia G, La Vignera S. Chronic prostatitis and its detrimental impact on sperm parameters: a systematic review and meta-analysis. J Endocrinol Invest. 2017; 5. 09. [Epub]. DOI: 10.1007/s40618-017-0684-0.
Article43. Adams JA, Galloway TS, Mondal D, Esteves SC, Mathews F. Effect of mobile telephones on sperm quality: a systematic review and meta-analysis. Environ Int. 2014; 70:106–112. PMID: 24927498.
Article44. Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur Urol. 2016; 70:635–645. PMID: 27113031.
Article45. R Bar-OrD Bar-OrLT Rael. Aytu Bioscience, Inc.Method and apparatus for measuring oxidation-reduction potential. United States patent. US 9,528,959. 2016. 12. 27.46. Rael LT, Bar-Or R, Kelly MT, Carrick MM, Bar-Or D. Assessment of oxidative stress in patients with an isolated traumatic brain injury using disposable electrochemical test strips. Electroanalysis. 2015; 27:2567–2573.
Article47. Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002; 30:620–650. PMID: 12512863.48. Victorin K, Hellström KG, Rylander R. Redox potential measurements for determining the disinfecting power of chlorinated water. J Hyg (Lond). 1972; 70:313–323. PMID: 4555890.
Article49. Bergendahl JA, Stevens L. Oxidation reduction potential as a measure of disinfection effectiveness for chlorination of wastewater. Environ Prog Sustain Energy. 2005; 24:214–222.
Article50. Ye Z, Wang S, Chen T, Gao W, Zhu S, He J, et al. Inactivation mechanism of escherichia coli induced by slightly acidic electrolyzed water. Sci Rep. 2017; 7:6279. PMID: 28740247.
Article51. McFarland MJ, Glarborg C, Ross MA. Chemical treatment of chelated metal finishing wastes. Water Environ Res. 2012; 84:2086–2089. PMID: 23342939.
Article52. Hjorth M, Pedersen CØ, Feilberg A. Redox potential as a means to control the treatment of slurry to lower HS emissions. Sensors (Basel). 2012; 12:5349–5362. PMID: 22778588.53. Agarwal A, Sharma R, Roychoudhury S, Du Plessis S, Sabanegh E. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril. 2016; 106:566–573.e10. PMID: 27260688.
Article54. Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online. 2017; 34:48–57. PMID: 27839743.
Article55. Agarwal A, Wang SM. Clinical relevance of oxidation-reduction potential in the evaluation of male infertility. Urology. 2017; 104:84–89. PMID: 28214572.
Article56. Agarwal A, Henkel R, Sharma R, Tadros N, Sabanegh E. Determination of seminal oxidation-reduction potential (ORP) as an easy and cost-effective clinical marker of male infertility. Andrologia. 2017; Forthcoming.
Article57. Agarwal A, Arafa M, Chandrakumar R, Majzoub A, AlSaid S, Elbardisi H. A multicenter study to evaluate oxidative stress by oxidation-reduction potential, a reliable and reproducible method. Andrology. 2017; 5:939–945. PMID: 28726302.
Article58. Arafa M, Agarwal A, AlSaid S, Majzoub A, Sharma R, Bjugstad KB, et al. Semen quality and infertility status can be identified through measures of oxidation-reduction potential. Andrologia. 2017; 8. 03. [Epub]. DOI: 10.1111/and.12881.
Article59. Agarwal A, Sharma R, Henkel R, Roychoudhury S, Sikka SC, duPlessis S, et al. Cumene hydroperoxide induced changes in oxidation-reduction potential in fresh and frozen seminal ejaculates. Andrologia. 2017; 3. 15. [Epub]. DOI: 10.1111/and.12796.
Article60. Shekarriz M, Thomas AJ Jr, Agarwal A. Effects of time and sperm concentration on reactive oxygen species formation in human semen. Arch Androl. 1995; 34:69–75. PMID: 7786090.
Article61. Du Plessis SS, Gokul S, Agarwal A. Semen hyperviscosity: causes, consequences, and cures. Front Biosci (Elite Ed). 2013; 5:224–231. PMID: 23276984.62. Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988; 9:367–376. PMID: 3215823.
Article63. Li Z, Zhou Y, Liu R, Lin H, Liu W, Xiao W, et al. Effects of semen processing on the generation of reactive oxygen species and mitochondrial membrane potential of human spermatozoa. Andrologia. 2012; 44:157–163. PMID: 21729130.
Article64. Agarwal A, Mulgund A, Sharma R, Sabanegh E. Mechanisms of oligozoospermia: an oxidative stress perspective. Syst Biol Reprod Med. 2014; 60:206–216. PMID: 24815996.
Article65. Roychoudhury C, Dorsey C, Choudhury BP, Kushal KK. Oxidation-reduction potential can help distinguish semen samples under oxidative stress. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.66. Elbardisi H, Arafa M, Agarwal A, AlSaid S, Majzoub A, Keyvanifard F, et al. Seminal fluid static oxidation reduction potential is lower in seminal fluid that meets WHO criteria for fertile men. Poster presented at: 32nd Annual European Society for Health and Reproduction Conference. 2016 Jul 3-6; Helsinki, Finland.67. Toor JS, Daniels L, Yafi FA, Hellstrom W, Sikka SC. Semen oxidative reduction potential (ORP) is related to abnormal semen parameters in male factor infertility. Poster presented at: 41st Annual America Society of Andrology Conference. 2016 Apr 2-5; New Orleans, LA, USA.68. Agarwal A, Elbardisi H, Arafa M, Okada H, Suzuki K, Homa S, et al. Multi-center evaluation of oxidation reduction potential assay in the infertile male. Poster presented at: 73rd Annual American Society for Reproductive Medicine Conference. 2017 Oct 28-Nov 1; San Antonio, TX, USA.69. Kovac JR, Smith RP, Cajipe M, Lamb DJ, Lipshultz LI. Men with a complete absence of normal sperm morphology exhibit high rates of success without assisted reproduction. Asian J Androl. 2017; 19:39–42. PMID: 27751992.
Article70. Shabtaie SA, Gerkowicz SA, Kohn TP, Ramasamy R. Role of abnormal sperm morphology in predicting pregnancy outcomes. Curr Urol Rep. 2016; 17:67. PMID: 27469478.
Article71. Franken DR. How accurate is sperm morphology as an indicator of sperm function? Andrologia. 2015; 47:720–723. PMID: 25130990.
Article72. Arafa M, Elbardisi H, Agarwal A, AlSaid S, Majzoub A, Al RumaihiK, et al. Oxidation-reduction potential: a new predictor for sperm morphology in infertile men. Poster presented at: 32nd Annual European Society for Health and Reproduction Conference. 2016 Jul 3-6; Helsinki, Finland.73. Ayaz A, Bjugstad KB, Bar-Or D, Armagan A. Infertile men have a redox balance that distinguishes them from fertile men. Poster presented at: 72nd Annual American Society for Reproductive Medicine Conference. 2016 Oct 15-19; Salt Lake City, UT, USA.74. Hamilton JA, Cissen M, Brandes M, Smeenk JM, de Bruin JP, Kremer JA, et al. Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system. Hum Reprod. 2015; 30:1110–1121. PMID: 25788568.
Article75. Borges E Jr, Setti AS, Braga DP, Figueira RC, Iaconelli A Jr. Total motile sperm count has a superior predictive value over the WHO 2010 cut-off values for the outcomes of intracytoplasmic sperm injection cycles. Andrology. 2016; 4:880–886. PMID: 27152971.
Article76. AlSaid S, Majzoub A, Arafa M, ElBardisi H, Agarwal A, AlRumaihi K. Oxidation-reduction potential: a valuable tool for male fertility evaluation. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.77. Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003; 79(Suppl 3):1597–1605. PMID: 12801566.
Article78. Simon L, Proutski I, Stevenson M, Jennings D, McManus J, Lutton D, et al. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod Biomed Online. 2013; 26:68–78. PMID: 23200202.
Article79. Lewis SE, John Aitken R, Conner SJ, Iuliis GD, Evenson DP, Henkel R, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online. 2013; 27:325–337. PMID: 23948450.
Article80. Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016; 5:935–950. PMID: 28078226.
Article81. Arafa M, ElBardisi H, Majzoub A, Al Said S, AlNawasra H, Khalafalla K, et al. Correlation of sperm DNA fragmentation and seminal oxidation-reduction potential in infertile men. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.82. Majzoub A, Arafa M, Elbardisi H, Al Said S, Agarwal A, Al Rumaihi K. Oxidation-reduction potential and sperm DNA fragmentation levels in sperm morphologic anomalies. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.83. Dorostghoal M, Kazeminejad SR, Shahbazian N, Pourmehdi M, Jabbari A. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia. 2017; 1. 26. [Epub]. DOI: 10.1111/and.12762.
Article84. Ionmmiello VM, Albani E, Di Rosa A, Marras A, Menduni F, Morreale G, et al. Ejaculate oxidative stress is related with sperm DNA fragmentation and round cells. Int J Endocrinol. 2015; 2015:321901. PMID: 25802519.85. Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017; 14:470–485. PMID: 28508879.
Article86. Ayaz A, Balaban B, Sikka S, Isiklar A, Tasdemir M, Urman B. Effect of seminal ORP value on embryo quality and clinical pregnancy rate. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.87. Agarwal A, Roychoudhury S, Esteves S, Rosas IM, Sharma R, Gupta S. Oxidation-reduction potential (ORP) of spermatazoa selected for intracytoplasmic sperm injection (ICSI) after exposure to polyvinylpyrrolidone (PVP) and hyaluronic acid (HA). Poster presented at: 32nd Annual European Society for Health and Reproduction Conference. 2016 Jul 3-6; Helsinki, Finland.88. Jensen CF, Ostergren P, Dupree JM, Ohl D, Sonksen J, Fode M. Varicocele and male infertility. Nat Rev Urol. 2017; 14:523–533. PMID: 28675168.
Article89. Agarwal A, Wang SM, Tadros N, Sabanegh E. Involvement of oxidation reduction potential in the pathophysiology of male infertility patients with varicocele. Poster presented at 112th Annual American Urological Association Conference. 2017 May 12-16; Boston, MA, USA.90. Arafa M, ElBardisi H, Majzoub A, AlSaid S, Jaber A, Khalafalla K, et al. Role of oxidation reduction potential in varicocele associated male infertility. Poster presented at 112th American Urological Association Conference. 2017 May 12-16; Boston, MA, USA.91. Agarwal A, Majzoub A, Roychoudhury S, Arafa MM. Oxidation reduction potential: a novel marker of varicocele pathophysiology. Poster presented at: 72nd Annual American Society for Reproductive Medicine Conference. 2016 Octr 15-19; Salt Lake City, UT, USA.92. Saleh R, Agarwal A. High levels of seminal oxidation-reduction potential (ORP) in infertile men with clinical varicocele. Poster presented at: 33rd Annual European Society for Health and Reproduction Conference. 2017 Jul 3-5; Geneva, Switzerland.93. Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, et al. Leukocytospermia is associated with increased reactive oxygen species production by human spermatazoa. Fertil Steril. 2002; 78:1215–1224. PMID: 12477515.94. Alvarez JG, Sharma RK, Ollero M, Saleh RA, Lopez MC, Thomas AJ Jr, et al. Increased DNA damage in sperm from leukocytospermic semen samples as determined by sperm chromatin structure assay. Fertil Steril. 2002; 78:319–329. PMID: 12137869.95. Hagan S, Khurana N, Chandra S, Abdel-Mageed AB, Mondal D, Hellstrom WJ, et al. Differential expression of novel biomarkers (TLR-2, TLR-4, COX-2, and Nrf-2) of inflammation and oxidative stress in semen of leukocytospermia patients. Andrology. 2015; 3:848–855. PMID: 26227162.
Article96. Sikka SC, Toor JS, Lucelia D, Yafi FA, Hellstrom W. Measurement of oxidation-reduction potential (ORP) as a newer tool indicative of oxidative stress in infertile men with leukocytospermia. Poster presented at: 41st Annual America Society of Andrology Conference. 2016 Apr 2-5; New Orleans, LA, USA.97. Kirby EW, Wiener LE, Rajanahally S, Crowell K, Coward RM. Undergoing varicocele repair before assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligospermic men with a varicocele: a systematic review and meta-analysis. Fertil Steril. 2016; 106:1338–1343. PMID: 27526630.
Article98. Majzoub A, Agarwal A. Antioxidant therapy in idiopathic oligoasthenoteratozoospermia. Indian J Urol. 2017; 33:207–214. PMID: 28717270.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Novel Approach to Improving the Reliability of Manual Semen Analysis: A Paradigm Shift in the Workup of Infertile Men
- Oxidative Stress: A Comprehensive Review of Biochemical, Molecular, and Genetic Aspects in the Pathogenesis and Management of Varicocele
- Aging increases oxidative stress in semen
- The correlation of Septin4 gene expression with sperm quality, DNA damage, and oxidative stress level in infertile patients
- Oxidative stress and endometriosis