Ann Dermatol.
2018 Apr;30(2):173-178. 10.5021/ad.2018.30.2.173.
Topical Tacrolimus for the Treatment of Atopic Dermatitis with Truncal Lesion
- Affiliations
-
- 1Department of Dermatology, Pusan National University School of Medicine, Busan, Korea.
- 2Department of Dermatology, School of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Dermatology, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 4Department of Dermatology, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Dermatology, Korea University College of Medicine, Seoul, Korea.
- 6Department of Dermatology, College of Medicine, Kyung Hee University, Seoul, Korea.
- 7Department of Dermatology, Konkuk University School of Medicine, Seoul, Korea.
- 8Department of Dermatology, Chungnam National University School of Medicine, Daejeon, Korea.
- 9Department of Dermatology, Sungkyunkwan University School of Medicine, Suwon, Korea.
- 10Department of Dermatology, School of Medicine, Chosun University, Gwangju, Korea.
- 11Department of Dermatology, University of Ulsan College of Medicine, Seoul, Korea.
- 12Department of Dermatology, Dongguk University College of Medicine, Gyeongju, Korea.
- 13Department of Dermatology, Gachon University College of Medicine, Incheon, Korea.
- 14Department of Dermatology, Kyungpook National University School of Medicine, Daegu, Korea.
- 15Department of Dermatology, Chonbuk National University Medical School, Jeonju, Korea.
- 16Department of Dermatology, Ajou University School of Medicine, Suwon, Korea.
- 17Department of Dermatology, Hallym University College of Medicine, Chuncheon, Korea. dermap@hanmail.net
- KMID: 2414678
- DOI: http://doi.org/10.5021/ad.2018.30.2.173
Abstract
- BACKGROUND
Topical tacrolimus is an effective anti-inflammatory therapy for acute and chronic states of atopic dermatitis (AD) in both adults and children. Topical tacrolimus has particular use at sensitive areas such as the face, anogenitals, and skin folds of neck and extremities. However, many AD patients also experience aggravated symptoms on trunk.
OBJECTIVE
The aim of this study was to investigate the efficacy and safety of topical tacrolimus for AD patients with truncal lesions.
METHODS
AD patients with truncal lesions who were aged ≥2 years were recruited from 20 centres in Korea. They received treatment with topical tacrolimus ointment twice daily during 4 weeks. The primary end point was change of the local eczema area and severity index (EASI) of the trunk from baseline to day 28. The secondary end points were changes in the patient global assessment (PGA) score and itch visual analogue scale (VAS) score of the trunk between baseline and day 28.
RESULTS
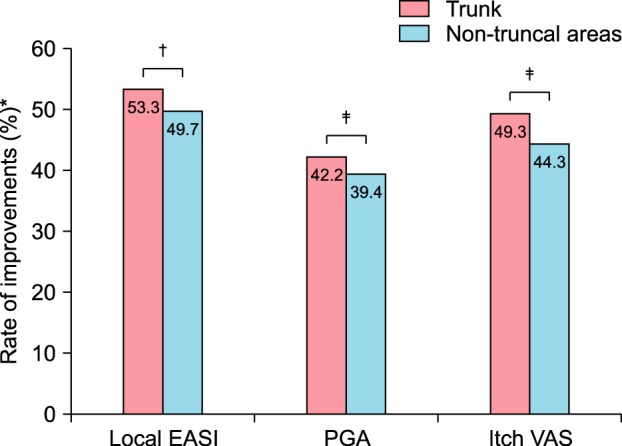
Two hundred and ninety-one patients were recruited, and 176 patients completed the full 4-week treatment course. By the end of the treatment, the mean local EASI of the trunk (2.2±4.71) was significantly decreased from that at baseline (4.71±4.03, p < 0.001). PGA (1.71±1.15) and itch VAS score of the trunk (2.61±2.19) on day 28 were also profoundly decreased compared with the baseline (2.96±1.07 and 5.15±2.47, respectively). No serious adverse events were observed during the study period.
CONCLUSION
Topical tacrolimus is an effective and safe therapy for truncal lesions in AD patients.
MeSH Terms
Figure
Reference
-
1. Won CH, Seo PG, Park YM, Yang JM, Lee KH, Sung KJ, et al. A multicenter trial of the efficacy and safety of 0.03% tacrolimus ointment for atopic dermatitis in Korea. J Dermatolog Treat. 2004; 15:30–34. PMID: 14754647.
Article2. Leung DY, Nicklas RA, Li JT, Bernstein IL, Blessing-Moore J, Boguniewicz M, et al. Disease management of atopic dermatitis: an updated practice parameter. Joint Task Force on Practice Parameters. Ann Allergy Asthma Immunol. 2004; 93(3 Suppl 2):S1–S21.3. Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014; 71:116–132. PMID: 24813302.4. Reitamo S, Van Leent EJ, Ho V, Harper J, Ruzicka T, Kalimo K, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone acetate ointment in children with atopic dermatitis. J Allergy Clin Immunol. 2002; 109:539–546. PMID: 11898004.
Article5. Hanifin JM, Rajka R. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.6. Long CC, Finlay AY, Averill RW. The rule of hand: 4 hand areas = 2 FTU = 1 g. Arch Dermatol. 1992; 128:1129–1130. PMID: 1497374.
Article7. Kalavala M, Mills CM, Long CC, Finlay AY. The fingertip unit: a practical guide to topical therapy in children. J Dermatolog Treat. 2007; 18:319–320. PMID: 17852630.
Article8. Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001; 10:11–18.9. Tofte S, Graeber M, Cherill R, Omoto M, Thurston M, Hanifin J, et al. Eczema area and severity index (EASI): a new tool to evaluate atopic dermatitis. J Eur Acad Dermatol Venereol. 1998; 11(Suppl 2):S197.
Article10. Murrell DF, Calvieri S, Ortonne JP, Ho VC, Weise-Riccardi S, Barbier N, et al. A randomized controlled trial of pimecrolimus cream 1% in adolescents and adults with head and neck atopic dermatitis and intolerant of, or dependent on, topical corticosteroids. Br J Dermatol. 2007; 157:954–959. PMID: 17935515.
Article11. Kawakami T, Soma Y, Morita E, Koro O, Yamamoto S, Nakamura K, et al. Safe and effective treatment of refractory facial lesions in atopic dermatitis using topical tacrolimus following corticosteroid discontinuation. Dermatology. 2001; 203:32–37. PMID: 11549797.
Article12. Hoeger PH, Lee KH, Jautova J, Wohlrab J, Guettner A, Mizutani G, et al. The treatment of facial atopic dermatitis in children who are intolerant of, or dependent on, topical corticosteroids: a randomized, controlled clinical trial. Br J Dermatol. 2009; 160:415–422. PMID: 19067708.
Article13. Reitamo S, Ortonne JP, Sand C, Cambazard F, Bieber T, Fölster-Holst R, et al. A multicentre, randomized, double-blind, controlled study of long-term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2005; 152:1282–1289. PMID: 15948994.
Article14. Moftah NH, Ibrahim SM, Wahba NH. Intense pulsed light versus photodynamic therapy using liposomal methylene blue gel for the treatment of truncal acne vulgaris: a comparative randomized split body study. Arch Dermatol Res. 2016; 308:263–268. PMID: 26993345.
Article15. Devaux S, Castela A, Archier E, Gallini A, Joly P, Misery L, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012; 26(Suppl 3):61–67. PMID: 22512682.
Article16. Jin H, Kim JM, Kim GW, Kim HS, Ko HC, Kim MB, et al. Inappropriate amounts of topical tacrolimus applied on Korean patients with eczema. J Dermatolog Treat. 2017; 28:327–331. PMID: 27588441.
Article17. Cury Martins J, Martins C, Aoki V, Gois AF, Ishii HA, da Silva EM. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev. 2015; (7):CD009864. PMID: 26132597.
Article18. Wollenberg A, Reitamo S, Atzori F, Lahfa M, Ruzicka T, Healy E, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008; 63:742–750.
Article19. Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011; 164:415–428. PMID: 20819086.
Article20. Tiwari A, Goel M, Pal P, Gohiya P. Topical-steroid-induced iatrogenic Cushing syndrome in the pediatric age group: a rare case report. Indian J Endocrinol Metab. 2013; 17(Suppl 1):S257–S258. PMID: 24251179.21. Gen R, Akbay E, Sezer K. Cushing syndrome caused by topical corticosteroid: a case report. Am J Med Sci. 2007; 333:173–174. PMID: 17496736.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Four Cases of Atopic Dermatitis with Topical Tacrolimus Therapy
- The Efficacy of Tapering Treatment with 0.1% Tacrolimus Ointment in Adult Patients with Atopic Dermatitis in Korea
- Efficacy of 0.1% Tacrolimus Ointment in Korean Patients with Atopic Dermatitis
- The Effect of Topical Tacrolimus in the Murine Contact Hypersensitivity and Dermatitis of Repeated Applications Induced by Diphenylcyclopropenone
- Tinea Incognito Caused by Application of 0.03% Tacrolimus (Protopic(R)) Ointment in Atopic Dermatitis Patient