J Gastric Cancer.
2018 Jun;18(2):142-151. 10.5230/jgc.2018.18.e14.
Modification of the TNM Staging System for Stage II/III Gastric Cancer Based on a Prognostic Single Patient Classifier Algorithm
- Affiliations
-
- 1Department of Surgery, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea. JHCHEONG@yuhs.ac
- 2Yonsei Biomedical Research Institute, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea.
- 3MediBio-Informatics Research Center, Novomics Co., Ltd., Seoul, Korea.
- 4Department of Radiology, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Biochemistry & Molecular Biology, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea.
- 6YUHS-KRIBB Medical Convergence Research Institute, Seoul, Korea.
- 7Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2414534
- DOI: http://doi.org/10.5230/jgc.2018.18.e14
Abstract
- PURPOSE
The modification of the cancer classification system aimed to improve the classical anatomy-based tumor, node, metastasis (TNM) staging by considering tumor biology, which is associated with patient prognosis, because such information provides additional precision and flexibility.
MATERIALS AND METHODS
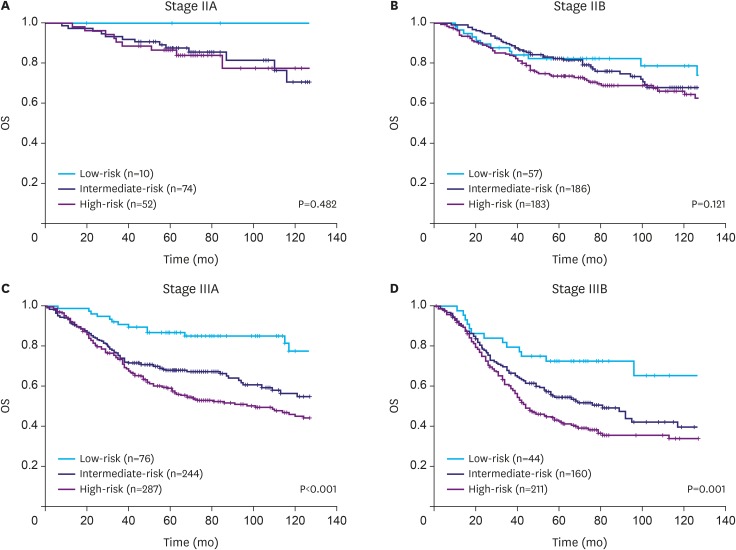
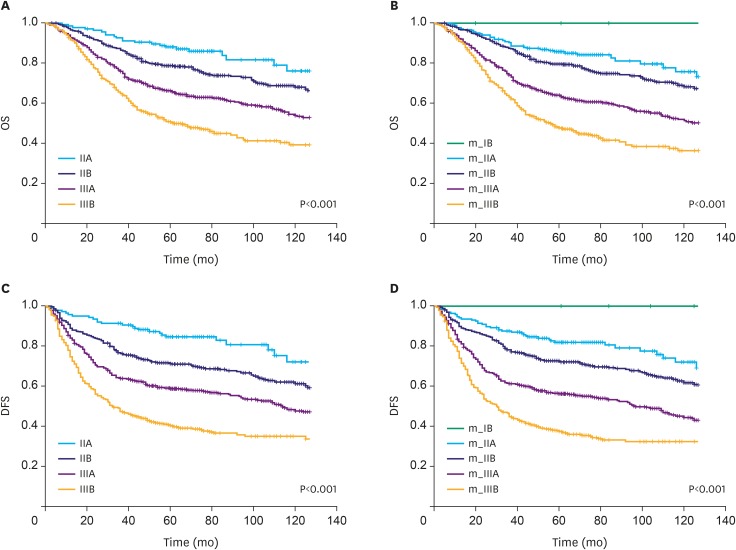
We previously developed an mRNA expression-based single patient classifier (SPC) algorithm that could predict the prognosis of patients with stage II/III gastric cancer. We also validated its utilization in clinical settings. The prognostic single patient classifier (pSPC) differentiates based on 3 prognostic groups (low-, intermediate-, and high-risk), and these groups were considered as independent prognostic factors along with TNM stages. We evaluated whether the modified TNM staging system based on the pSPC has a better prognostic performance than the TNM 8th edition staging system. The data of 652 patients who underwent gastrectomy with curative intent for gastric cancer between 2000 and 2004 were evaluated. Furthermore, 2 other cohorts (n=307 and 625) from a previous study were assessed. Thus, 1,584 patients were included in the analysis. To modify the TNM staging system, one-grade down-staging was applied to low-risk patients according to the pSPC in the TNM 8th edition staging system; for intermediate- and high-risk groups, the modified TNM and TNM 8th edition staging systems were identical.
RESULTS
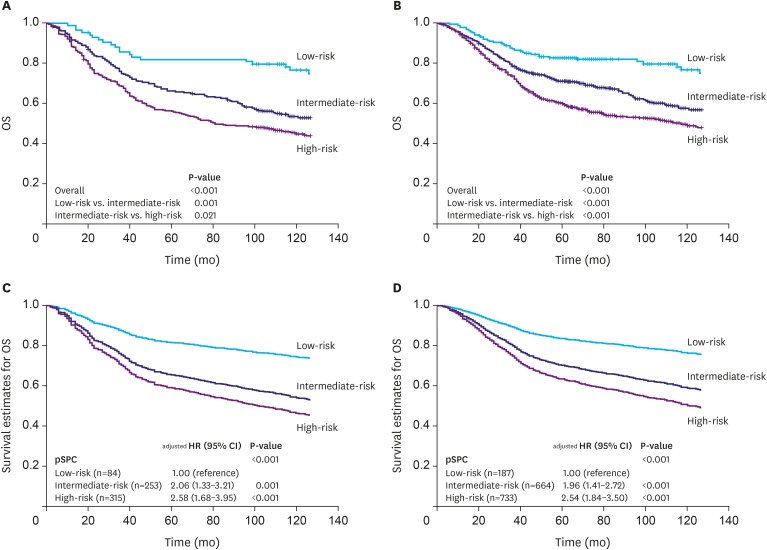
Among the 1,584 patients, 187 (11.8%), 664 (41.9%), and 733 (46.3%) were classified into the low-, intermediate-, and high-risk groups, respectively, according to the pSPC. pSPC prognoses and survival curves of the overall population were well stratified, and the TNM stage-adjusted hazard ratios of the intermediate- and high-risk groups were 1.96 (95% confidence interval [CI], 1.41-2.72; P < 0.001) and 2.54 (95% CI, 1.84-3.50; P < 0.001), respectively. Using Harrell's C-index, the prognostic performance of the modified TNM system was evaluated, and the results showed that its prognostic performance was better than that of the TNM 8th edition staging system in terms of overall survival (0.635 vs. 0.620, P < 0.001).
CONCLUSIONS
The pSPC-modified TNM staging is an alternative staging system for stage II/III gastric cancer.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Ten Thousand Consecutive Gastrectomies for Gastric Cancer: Perspectives of a Master Surgeon
Yoon Young Choi, Minah Cho, In Gyu Kwon, Taeil Son, Hyoung-Il Kim, Seung Ho Choi, Jae-Ho Cheong, Woo Jin Hyung
Yonsei Med J. 2019;60(3):235-242. doi: 10.3349/ymj.2019.60.3.235.Clinical Implementation of Precision Medicine in Gastric Cancer
Jaewook Jeon, Jae-Ho Cheong
J Gastric Cancer. 2019;19(3):235-253. doi: 10.5230/jgc.2019.19.e25.
Reference
-
1. Union for International Cancer Control. What is TNM? [Internet]. place unknown: Union for International Cancer Control;cited 2018 May 28. Available from: https://www.uicc.org/resources/tnm.2. Brierley JD, Catton PA, O'Sullivan B, Dancey JE, Dowling AJ, Irish JC, et al. Accuracy of recorded tumor, node, and metastasis stage in a comprehensive cancer center. J Clin Oncol. 2002; 20:413–419. PMID: 11786568.
Article3. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed. Basel: Springer;2017.4. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID: 25220842.
Article5. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014; 513:202–209. PMID: 25079317.6. Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015; 21:449–456. PMID: 25894828.
Article7. Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011; 141:476–485. 485.e1–485.e11. PMID: 21684283.
Article8. Choi YY, Cheong JH. Beyond precision surgery: molecularly motivated precision care for gastric cancer. Eur J Surg Oncol. 2017; 43:856–864. PMID: 28330821.
Article9. Cheong JH, Yang HK, Kim H, Kim WH, Kim YW, Kook MC, et al. Predictive test for chemotherapy response in resectable gastric cancer: a multi-cohort, retrospective analysis. Lancet Oncol. 2018; 19:629–638. PMID: 29567071.
Article10. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:1389–1396. PMID: 25439693.11. Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984; 3:143–152. PMID: 6463451.
Article12. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.
Article13. Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol. 2017; 3:1197–1203. PMID: 28241187.14. Choi YY, Kim H, Yang HK, Kim WH, Kim YW, Kook MC, et al. Clinical impact of microsatellite instability in patients with stage II and III gastric cancer: results from the CLASSIC trial. J Clin Oncol. 2017.
Article15. Nakajima T, Nashimoto A, Kitamura M, Kito T, Iwanaga T, Okabayashi K, et al. Adjuvant mitomycin and fluorouracil followed by oral uracil plus tegafur in serosa-negative gastric cancer: a randomised trial. Lancet. 1999; 354:273–277. PMID: 10440302.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Value of the Anatomic Region of Metastatic Lymph Nodes in the Current TNM Staging of Gastric Cancer
- Comparison of the Differences in Survival Rates between the 7th and 8th Editions of the AJCC TNM Staging System for Gastric Adenocarcinoma: a Single-Institution Study of 5,507 Patients in Korea
- Evaluation of the New UICC Staging System for Gastric Carcinoma
- Analysis of Prognostic Factors in 448 Gastric Cancer Patients Treated with a Gastric Resection
- Individualized Cutoff Value of the Preoperative Carcinoembryonic Antigen Level is Necessary for Optimal Use as a Prognostic Marker