Korean J Physiol Pharmacol.
2018 Jul;22(4):419-425. 10.4196/kjpp.2018.22.4.419.
The effect of µ-opioid receptor activation on GABAergic neurons in the spinal dorsal horn
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. sangjkim@snu.ac.kr
- 2Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Neuroscience Research Institute, Seoul National University College of Medicine, Seoul 03080, Korea.
- 4Department of Physiology, College of Korean Medicine, Gachon University, Seongnam 13120, Korea.
- KMID: 2414266
- DOI: http://doi.org/10.4196/kjpp.2018.22.4.419
Abstract
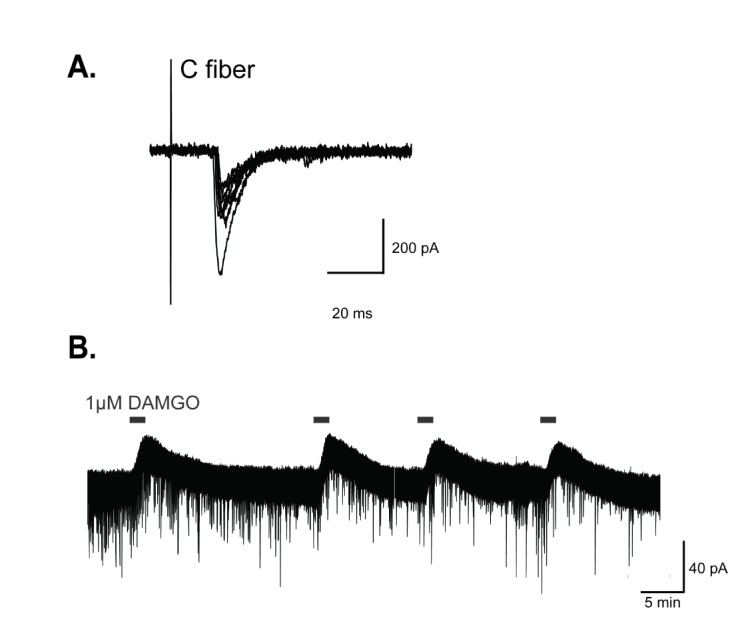
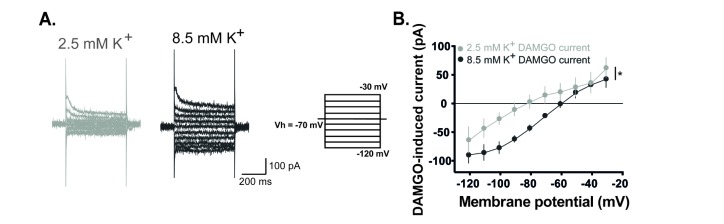
- The superficial dorsal horn of the spinal cord plays an important role in pain transmission and opioid activity. Several studies have demonstrated that opioids modulate pain transmission, and the activation of µ-opioid receptors (MORs) by opioids contributes to analgesic effects in the spinal cord. However, the effect of the activation of MORs on GABAergic interneurons and the contribution to the analgesic effect are much less clear. In this study, using transgenic mice, which allow the identification of GABAergic interneurons, we investigated how the activation of MORs affects the excitability of GABAergic interneurons and synaptic transmission between primary nociceptive afferent and GABAergic interneurons. We found that a selective µ-opioid agonist, [D-Ala², NMe-Pheâ´, Gly-ol]-enkephanlin (DAMGO), induced an outward current mediated by K⺠channels in GABAergic interneurons. In addition, DAMGO reduced the amplitude of evoked excitatory postsynaptic currents (EPSCs) of GABAergic interneurons which receive monosynaptic inputs from primary nociceptive C fibers. Taken together, we found that DAMGO reduced the excitability of GABAergic interneurons and synaptic transmission between primary nociceptive C fibers and GABAergic interneurons. These results suggest one possibility that suppression of GABAergic interneurons by DMAGO may reduce the inhibition on secondary GABAergic interneurons, which increase the inhibition of the secondary GABAergic interneurons to excitatory neurons in the spinal dorsal horn. In this circumstance, the sum of excitation of the entire spinal network will control the pain transmission.
Keyword
MeSH Terms
-
Analgesics, Opioid
Animals
Enkephalin, Ala(2)-MePhe(4)-Gly(5)-
Excitatory Postsynaptic Potentials
GABAergic Neurons*
Interneurons
Mice
Mice, Transgenic
Nerve Fibers, Unmyelinated
Neurons
Spinal Cord
Spinal Cord Dorsal Horn*
Substantia Gelatinosa
Synaptic Transmission
Analgesics, Opioid
Enkephalin, Ala(2)-MePhe(4)-Gly(5)-
Figure
Reference
-
1. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009; 139:267–284. PMID: 19837031.
Article2. Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986; 234:358–361. PMID: 3764416.
Article3. Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978; 177:417–434. PMID: 412881.
Article4. Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979; 186:117–131. PMID: 447880.
Article5. Zeilhofer HU, Wildner H, Yévenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012; 92:193–235. PMID: 22298656.
Article6. Spike RC, Todd AJ, Johnston HM. Coexistence of NADPH diaphorase with GABA, glycine, and acetylcholine in rat spinal cord. J Comp Neurol. 1993; 335:320–333. PMID: 8227522.
Article7. Todd AJ, McKenzie J. GABA-immunoreactive neurons in the dorsal horn of the rat spinal cord. Neuroscience. 1989; 31:799–806. PMID: 2594201.
Article8. Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990; 296:496–505. PMID: 2358549.
Article9. Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I-III of the mammalian spinal dorsal horn. Prog Neurobiol. 1993; 41:609–645. PMID: 7904359.
Article10. Furue H, Katafuchi T, Yoshimura M. Sensory processing and functional reorganization of sensory transmission under pathological conditions in the spinal dorsal horn. Neurosci Res. 2004; 48:361–368. PMID: 15041189.
Article11. Cui L, Kim YR, Kim HY, Lee SC, Shin HS, Szabó G, Erdélyi F, Kim J, Kim SJ. Modulation of synaptic transmission from primary afferents to spinal substantia gelatinosa neurons by group III mGluRs in GAD65-EGFP transgenic mice. J Neurophysiol. 2011; 105:1102–1111. PMID: 21177998.
Article12. Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003; 23:8752–8758. PMID: 14507975.
Article13. Yasaka T, Kato G, Furue H, Rashid MH, Sonohata M, Tamae A, Murata Y, Masuko S, Yoshimura M. Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro. J Physiol. 2007; 581:603–618. PMID: 17347278.14. Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GAB-Aergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002; 22:6724–6731. PMID: 12151551.
Article15. Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006; 26:1833–1843. PMID: 16467532.
Article16. Fürst S. Transmitters involved in antinociception in the spinal cord. Brain Res Bull. 1999; 48:129–141. PMID: 10230704.
Article17. Cho PS, Lee HK, Lee SH, Im JZ, Jung SJ. DAMGO modulates two-pore domain K+ channels in the substantia gelatinosa neurons of rat spinal cord. Korean J Physiol Pharmacol. 2016; 20:525–531. PMID: 27610039.18. Wu SY, Ohtubo Y, Brailoiu GC, Dun NJ. Effects of endomorphin on substantia gelatinosa neurons in rat spinal cord slices. Br J Pharmacol. 2003; 140:1088–1096. PMID: 14530213.
Article19. Omote K, Kitahata LM, Collins JG, Nakatani K, Nakagawa I. The antinociceptive role of mu- and delta-opiate receptors and their interactions in the spinal dorsal horn of cats. Anesth Analg. 1990; 71:23–28. PMID: 1973027.20. Czlonkowski A, Costa T, Przewlocki R, Pasi A, Herz A. Opiate receptor binding sites in human spinal cord. Brain Res. 1983; 267:392–396. PMID: 6307472.21. Chang KJ, Cuatrecasas P. Multiple opiate receptors. Enkephalins and morphine bind to receptors of different specificity. J Biol Chem. 1979; 254:2610–2618. PMID: 218947.
Article22. Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995; 15:3328–3341. PMID: 7751913.
Article23. Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990; 521:15–22. PMID: 2169958.24. Marker CL, Luján R, Colón J, Wickman K. Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J Neurosci. 2006; 26:12251–12259. PMID: 17122050.
Article25. Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000; 40:389–430. PMID: 10836142.
Article26. Yaksh TL. Pharmacology and mechanisms of opioid analgesic activity. Acta Anaesthesiol Scand. 1997; 41:94–111. PMID: 9061092.
Article27. Glaum SR, Miller RJ, Hammond DL. Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina II neurons of adult rat spinal cord. J Neurosci. 1994; 14:4965–4971. PMID: 8046463.
Article28. Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J Physiol. 1999; 518:803–813. PMID: 10420016.
Article29. Yaksh TL. Opiate receptors for behavioral analgesia resemble those related to the depression of spinal nociceptive neurons. Science. 1978; 199:1231–1233. PMID: 204008.
Article30. Chen J, Sandkühler J. Induction of homosynaptic long-term depression at spinal synapses of sensory a delta-fibers requires activation of metabotropic glutamate receptors. Neuroscience. 2000; 98:141–148. PMID: 10858620.31. Ruscheweyh R, Sandkühler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol. 2002; 541:231–244. PMID: 12015432.32. Kerchner GA, Zhuo M. Presynaptic suppression of dorsal horn inhibitory transmission by mu-opioid receptors. J Neurophysiol. 2002; 88:520–522. PMID: 12091574.33. Schneider SP, Eckert WA 3rd, Light AR. Opioid-activated postsynaptic, inward rectifying potassium currents in whole cell recordings in substantia gelatinosa neurons. J Neurophysiol. 1998; 80:2954–2962. PMID: 9862898.
Article34. Kemp T, Spike RC, Watt C, Todd AJ. The mu-opioid receptor (MOR1) is mainly restricted to neurons that do not contain GABA or glycine in the superficial dorsal horn of the rat spinal cord. Neuroscience. 1996; 75:1231–1238. PMID: 8938756.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Suppression by Microinjection of Bicuculline into Brain Stem Nuclei of Dorsal Horn Neuron Responsiveness in Neuropathic Rats
- A Role of Glutamate and GABA Receptors in the Generation of Formalin-induced Impulse Firing of Spinal Dorsal Horn Neurons in the Rat
- Loss of the Spinal GABAergic System Is Involved in Chronic Central Pain Following a Spinal Cord Injury; Behavioral and Electrophysiological Evidences
- Spinal and Peripheral GABA-A and B Receptor Agonists for the Alleviation of Mechanical Hypersensitivity following Compressive Nerve Injury in the Rat
- The expression of corticotropin-releasing factor and its receptors in the spinal cord and dorsal root ganglion in a rat model of neuropathic pain