Korean J Physiol Pharmacol.
2018 Jul;22(4):391-398. 10.4196/kjpp.2018.22.4.391.
Anti-inflammatory and utero-relaxant effect of α-bisabolol on the pregnant human uterus
- Affiliations
-
- 1Ãrea Académica de Medicina del Instituto de Ciencias de la Salud. Universidad Autónoma del Estado de Hidalgo, Pachuca, Hidalgo, México. mario_i_ortiz@hotmail.com
- 2Hospital General de los SSH, Pachuca, Hidalgo, Mexico.
- KMID: 2414263
- DOI: http://doi.org/10.4196/kjpp.2018.22.4.391
Abstract
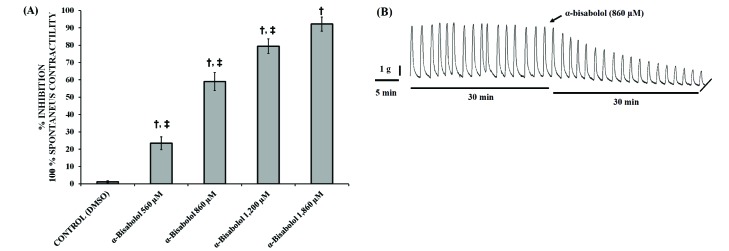
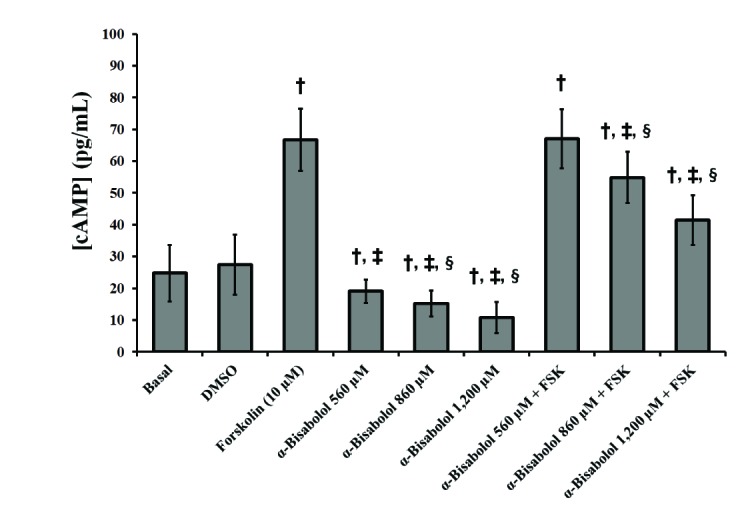
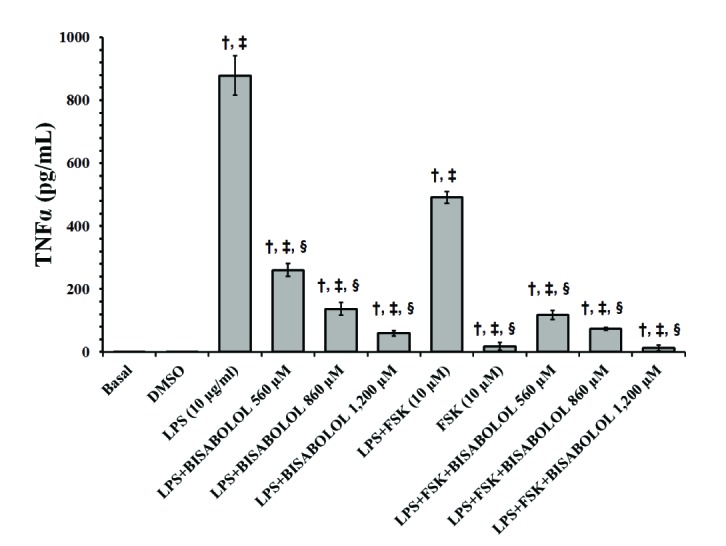
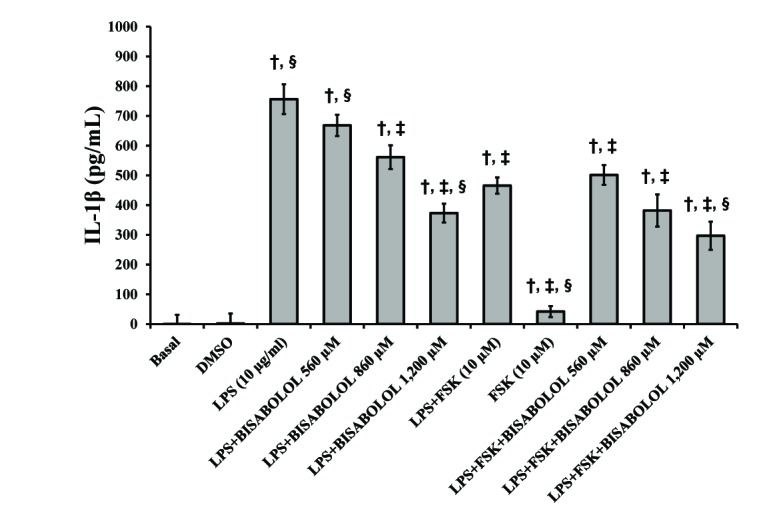
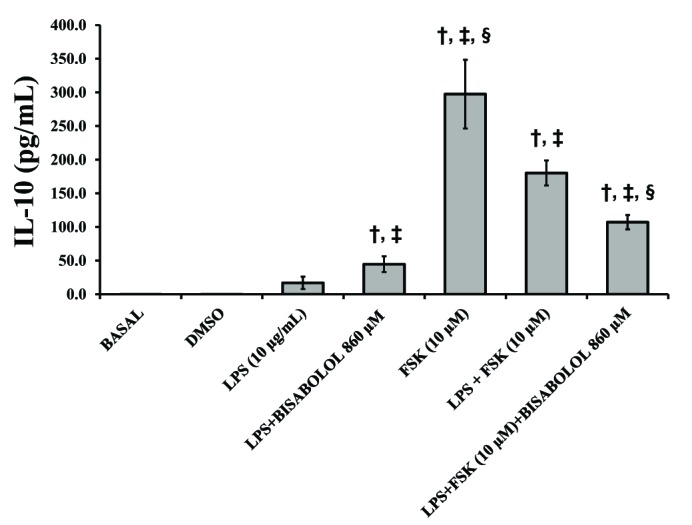
- The aim of this study was to evaluate the in vitro anti-inflammatory and utero-relaxant effect of α-bisabolol on the pregnant human myometrium. Samples from the pregnant human myometrium were used in functional tests to evaluate the inhibitory effect of α-bisabolol (560, 860, 1,200 and 1,860 µM) on spontaneous myometrial contractions. The intracellular cyclic adenosine monophosphate (cAMP) levels generated in response to α-bisabolol in human myometrial homogenates were measured by ELISA. The anti-inflammatory effect of α-bisabolol was determined through the measurement of two pro-inflammatory cytokines, tumor necrosis factor-α (TNFα) and interleukin (IL)-1β, and the anti-inflammatory cytokine IL-10, in pregnant human myometrial explants stimulated with lipopolysaccharide (LPS). Forskolin was used as a positive control to evaluate the cAMP and cytokine levels. α-Bisabolol was found to induce a significant inhibition of spontaneous myometrial contractions at the highest concentration level (p < 0.05). α-Bisabolol caused a concentration-dependent decrease in myometrial cAMP levels (p < 0.05) and a concentration-dependent decrease in LPS-induced TNFα and IL-1β production, while IL-10 production did not increase significantly (p>0.05). The anti-inflammatory and utero-relaxant effects induced by α-bisabolol were not associated with an increase in cAMP levels in pregnant human myometrial samples. These properties place α-bisabolol as a potentially safe and effective adjuvant agent in cases of preterm birth, an area of pharmacological treatment that requires urgent improvement.
Keyword
MeSH Terms
-
Adenosine Monophosphate
Animals
Colforsin
Cytokines
Enzyme-Linked Immunosorbent Assay
Female
Humans*
Immunomodulation
In Vitro Techniques
Inflammation
Interleukin-10
Interleukins
Mice
Myometrium
Necrosis
Obstetric Labor, Premature
Pregnancy
Premature Birth
Uterine Contraction
Uterus*
Adenosine Monophosphate
Colforsin
Cytokines
Interleukin-10
Interleukins
Figure
Reference
-
1. Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SG. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci. 2010; 17:619–628. PMID: 20581349.
Article2. Burdet J, Rubio AP, Salazar AI, Ribeiro ML, Ibarra C, Franchi AM. Inflammation, infection and preterm birth. Curr Pharm Des. 2014; 20:4741–4748. PMID: 24588830.
Article3. Muñoz-Pérez VM, Fernández-Martínez E, Ponce-Monter H, Ortiz MI. Relaxant and anti-inflammatory effect of two thalidomide analogs as PDE-4 inhibitors in pregnant rat uterus. Korean J Physiol Pharmacol. 2017; 21:429–437. PMID: 28706457.
Article4. Hubinont C, Debieve F. Prevention of preterm labour: 2011 update on tocolysis. J Pregnancy. 2011; 2011:941057. PMID: 22175022.
Article5. de Heus R, Mol BW, Erwich JJ, van Geijn HP, Gyselaers WJ, Hanssens M, Härmark L, van Holsbeke CD, Duvekot JJ, Schobben FF, Wolf H, Visser GH. Adverse drug reactions to tocolytic treatment for preterm labour: prospective cohort study. BMJ. 2009; 338:b744. PMID: 19264820.
Article6. Abitayeh G, Tsatsaris V, Goffinet F, Cabrol D. New tocolytic agents. Eur Clinics Obstet Gynecol. 2005; 1:29–35.
Article7. Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med. 2012; 17:12–19. PMID: 21944863.
Article8. Rocha NF, Oliveira GV, Araújo FY, Rios ER, Carvalho AM, Vasconcelos LF, Macêdo DS, Soares PM, Sousa DP, Sousa FC. (−)-α-Bisabolol-induced gastroprotection is associated with reduction in lipid peroxidation, superoxide dismutase activity and neutrophil migration. Eur J Pharm Sci. 2011; 44:455–461. PMID: 21924353.
Article9. de Siqueira RJ, Freire WB, Vasconcelos-Silva AA, Fonseca-Magalhães PA, Lima FJ, Brito TS, Mourão LT, Ribeiro RA, Lahlou S, Magalhães PJ. In-vitro characterization of the pharmacological effects induced by (-)-α-bisabolol in rat smooth muscle preparations. Can J Physiol Pharmacol. 2012; 90:23–35. PMID: 22171824.
Article10. Ko SG, Yin CS, Du B, Kim KH. Herbal medicines for inflammatory diseases 2016. Mediators Inflamm. 2016; 2016:8270323. PMID: 27847408.
Article11. Jeon JS, Kim HT, Kim MG, Oh MS, Hong SR, Yoon MH, Shin HC, Shim JH, Afifi NA, Hacımüftüoğlu A, El-Aty AMA. Simultaneous detection of glabridin, (−)-α-bisabolol, and ascorbyl tetraisopalmitate in whitening cosmetic creams using HPLC-PAD. Chromatographia. 2016; 79:851–860.
Article12. de Siqueira RJ, Ribeiro-Filho HV, Freire RS, Cosker F, Freire WB, Vasconcelos-Silva AA, Soares MA, Lahlou S, Magalhães PJ. (−)-α-Bisabolol inhibits preferentially electromechanical coupling on rat isolated arteries. Vascul Pharmacol. 2014; 63:37–45. PMID: 25128618.
Article13. Fernández-Martínez E, Ponce-Monter H, Soria-Jasso LE, Ortiz MI, Arias-Montaño JA, Barragán-Ramírez G, Mayén-García C. Inhibition of uterine contractility by thalidomide analogs via phosphodiesterase-4 inhibition and calcium entry blockade. Molecules. 2016; 21:E1332. PMID: 27739411.
Article14. Oger S, Méhats C, Barnette MS, Ferré F, Cabrol D, Leroy MJ. Anti-inflammatory and utero-relaxant effects in human myometrium of new generation phosphodiesterase 4 inhibitors. Biol Reprod. 2004; 70:458–464. PMID: 14561639.
Article15. Kinney MV, Lawn JE, Howson CP, Belizan J. 15 Million preterm births annually: what has changed this year? Reprod Health. 2012; 9:28. PMID: 23148557.
Article16. Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol. 2017; 41:387–391. PMID: 28865982.
Article17. Rezaeizadeh G, Hantoushzadeh S, Ghiasi S, Nikfar S, Abdollahi M. A systematic review of the uterine relaxant effect of herbal sources. Curr Pharm Biotechnol. 2016; 17:934–948. PMID: 27396394.
Article18. Tribe RM. Regulation of human myometrial contractility during pregnancy and labour: are calcium homeostatic pathways important? Exp Physiol. 2001; 86:247–254. PMID: 11429641.
Article19. Garfield RE, Maner WL. Physiology and electrical activity of uterine contractions. Semin Cell Dev Biol. 2007; 18:289–295. PMID: 17659954.
Article20. Pehlivanoğlu B, Bayrak S, Doğan M. A close look at the contraction and relaxation of the myometrium; the role of calcium. J Turk Ger Gynecol Assoc. 2013; 14:230–234. PMID: 24592112.21. Luckas MJ, Taggart MJ, Wray S. Intracellular calcium stores and agonist-induced contractions in isolated human myometrium. Am J Obstet Gynecol. 1999; 181:468–476. PMID: 10454702.
Article22. Roberts RE, Allen S, Chang AP, Henderson H, Hobson GC, Karania B, Morgan KN, Pek AS, Raghvani K, Shee CY, Shikotra J, Street E, Abbas Z, Ellis K, Heer JK, Alexander SP. Distinct mechanisms of relaxation to bioactive components from chamomile species in porcine isolated blood vessels. Toxicol Appl Pharmacol. 2013; 272:797–805. PMID: 23845591.
Article23. Sassone-Corsi P. The cyclic AMP pathway. Cold Spring Harb Perspect Biol. 2012; 4:a011148. PMID: 23209152.
Article24. Taskén K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004; 84:137–167. PMID: 14715913.25. Brenner S, Prösch S, Schenke-Layland K, Riese U, Gausmann U, Platzer C. cAMP-induced Interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J Biol Chem. 2003; 278:5597–5604. PMID: 12493739.
Article26. Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol. 2003; 23:305–314. PMID: 12825829.27. Choi YH, Kim GY, Lee HH. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des Devel Ther. 2014; 8:1941–1953.28. Cha JY, Jung JY, Jung JY, Lee JR, Cho IJ, Ku SK, Byun SH, Ahn YT, Lee CW, Kim SC, An WG. Inhibitory effects of traditional herbal formula pyungwi-san on inflammatory response in vitro and in vivo. Evid Based Complement Alternat Med. 2013; 2013:630198. PMID: 23533508.29. Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. 2014; 11:571–581. PMID: 24954221.
Article30. Jantan I, Ahmad W, Bukhari SN. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front Plant Sci. 2015; 6:655. PMID: 26379683.
Article31. Kiemer AK, Müller C, Vollmar AM. Inhibition of LPS-induced nitric oxide and TNF-alpha production by alpha-lipoic acid in rat Kupffer cells and in RAW 264.7 murine macrophages. Immunol Cell Biol. 2002; 80:550–557. PMID: 12406389.32. Saad B, Abouatta BS, Basha W, Hmade A, Kmail A, Khasib S, Said O. Hypericum triquetrifolium-derived factors downregulate the production levels of LPS-induced nitric oxide and tumor necrosis factor-α in THP-1 cells. Evid Based Complement Alternat Med. 2011; 2011:586470. PMID: 18955363.33. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012; 32:23–63. PMID: 22428854.
Article34. Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010; 10:170–181. PMID: 20154735.
Article35. Kelly A, Lynch A, Vereker E, Nolan Y, Queenan P, Whittaker E, O'Neill LA, Lynch MA. The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term potentiation. A role for JNK. J Biol Chem. 2001; 276:45564–45572. PMID: 11581275.36. Oger S, Méhats C, Dallot E, Ferré F, Leroy MJ. Interleukin-1beta induces phosphodiesterase 4B2 expression in human myometrial cells through a prostaglandin E2- and cyclic adenosine 3′,5′-monophosphate-dependent pathway. J Clin Endocrinol Metab. 2002; 87:5524–5531. PMID: 12466348.37. D'Almeida APL, Pacheco de Oliveira MT, de Souza ÉT, de Sá Coutinho D, Ciambarella BT, Gomes CR, Terroso T, Guterres SS, Pohlmann AR, Silva PM, Martins MA, Bernardi A. α-Bisabolol-loaded lipid-core nanocapsules reduce lipopolysaccharide-induced pulmonary inflammation in mice. Int J Nanomedicine. 2017; 12:4479–4491. PMID: 28684908.38. Roh E, Yun CY, Young Yun J, Park D, Doo Kim N, Yeon Hwang B, Jung SH, Park SK, Kim YB, Han SB, Kim Y. cAMP-binding site of PKA as a molecular target of bisabolangelone against melanocyte-specific hyperpigmented disorder. J Invest Dermatol. 2013; 133:1072–1079. PMID: 23254773.
Article39. Kim S, Lee J, Jung E, Huh S, Park JO, Lee JW, Byun SY, Park D. Mechanisms of depigmentation by alpha-bisabolol. J Dermatol Sci. 2008; 52:219–222. PMID: 18692366.40. Avni D, Ernst O, Philosoph A, Zor T. Role of CREB in modulation of TNFalpha and IL-10 expression in LPS-stimulated RAW264.7 macrophages. Mol Immunol. 2010; 47:1396–1403. PMID: 20303596.41. Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010; 185:6413–6419. PMID: 21084670.
Article42. Shima E, Katsube M, Kato T, Kitagawa M, Hato F, Hino M, Takahashi T, Fujita H, Kitagawa S. Calcium channel blockers suppress cytokine-induced activation of human neutrophils. Am J Hypertens. 2008; 21:78–84. PMID: 18091748.
Article43. Barreto RSS, Quintans JSS, Amarante RKL, Nascimento TS, Amarante RS, Barreto AS, Pereira EWM, Duarte MC, Coutinho HDM, Menezes IRA, Zengin G, Aktumsek A, Quintans-Júnior LJ. Evidence for the involvement of TNF-α and IL-1β in the antinociceptive and anti-inflammatory activity of Stachys lavandulifolia Vahl. (Lamiaceae) essential oil and (−)-α-bisabolol, its main compound, in mice. J Ethnopharmacol. 2016; 191:9–18. PMID: 27292196.
Article44. Marongiu L, Donini M, Bovi M, Perduca M, Vivian F, Romeo A, Mariotto S, Monaco HL, Dusi S. The inclusion into PLGA nanoparticles enables α-bisabolol to efficiently inhibit the human dendritic cell pro-inflammatory activity. J Nanopart Res. 2014; 16:2554.
Article45. Corpas-López V, Morillas-Márquez F, Navarro-Moll MC, Merino-Espinosa G, Díaz-Sáez V, Martín-Sánchez J. (−)-α-Bisabolol, a promising oral compound for the treatment of visceral leishmaniasis. J Nat Prod. 2015; 78:1202–1207. PMID: 26076227.
Article46. Andersen FA. Final report on the safety assessment of Bisabolol. Int J Toxicol. 1999; 18(3 suppl):33–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relaxant and anti-inflammatory effect of two thalidomide analogs as PDE-4 inhibitors in pregnant rat uterus
- A Case of the Torsion of the Term Pregnant Uterus with a Transverse Lie of the Fetus
- Effect of Oxytocin on the Succinylcholine Induced Neuromuscular Blockade
- Contractile Responses to Endothelins in Isolated Arteries from Human Uterus
- Distribution of T- & B-cell Series and Macrophages in the Peripheral Blood and the Utero-placental Interface of Pregnant Mice