Lab Med Online.
2018 Jul;8(3):77-86. 10.3343/lmo.2018.8.3.77.
Evaluation of the Self-Testing Blood Glucose Monitoring System GlucoDr.S According to ISO 15197:2013 Guidelines
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea. khlee59023@gmail.com
- 2Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Department of Biomedical Laboratory Science, Shinhan University, Uijeongbu, Korea.
- KMID: 2414128
- DOI: http://doi.org/10.3343/lmo.2018.8.3.77
Abstract
- BACKGROUND
The performance of the self-monitoring of blood glucose in patients with diabetes should be properly evaluated to ensure strict glycemic control. This study evaluated the self-testing Blood Glucose Monitoring System GlucoDr.Sâ„¢ (All Medicus Co., Ltd., Korea).
METHODS
This study recruited 120 patients. Use of the glucometer was evaluated according to ISO 15197:2013 guidelines. The YSI 2300 STAT PLUS Glucose Analyzer (YSI Life Sciences, USA) was used as the reference device.
RESULTS
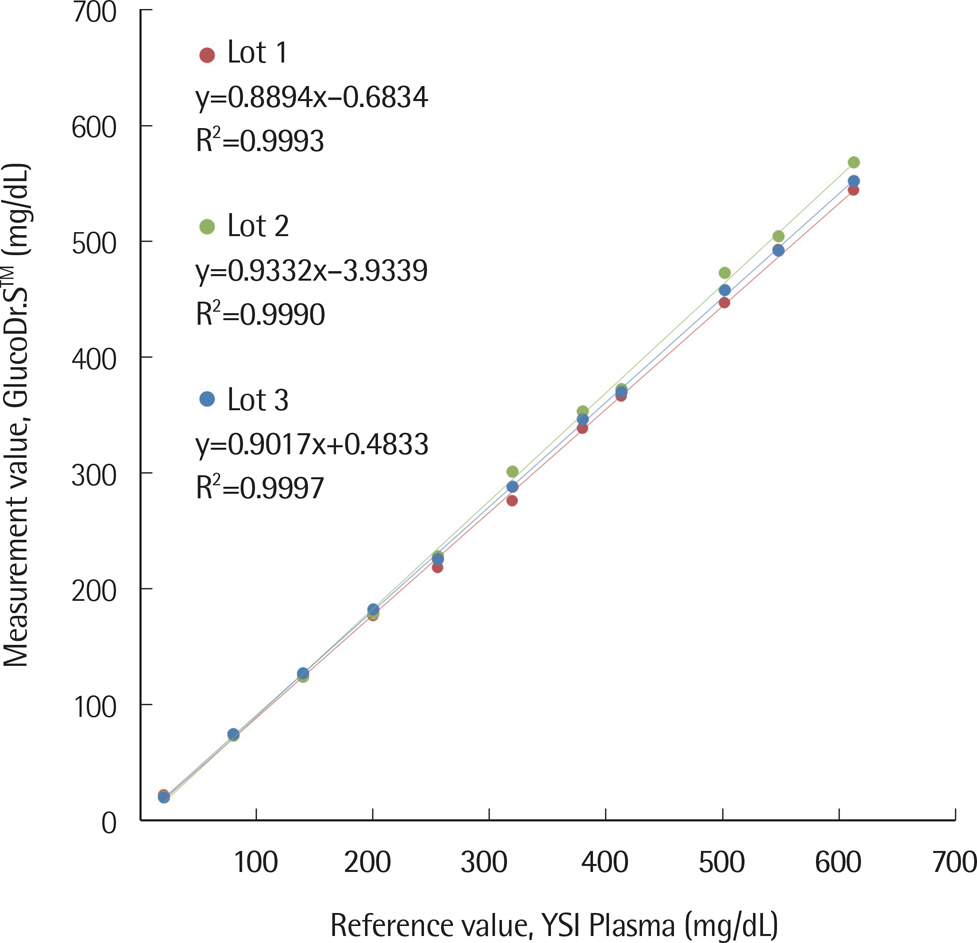
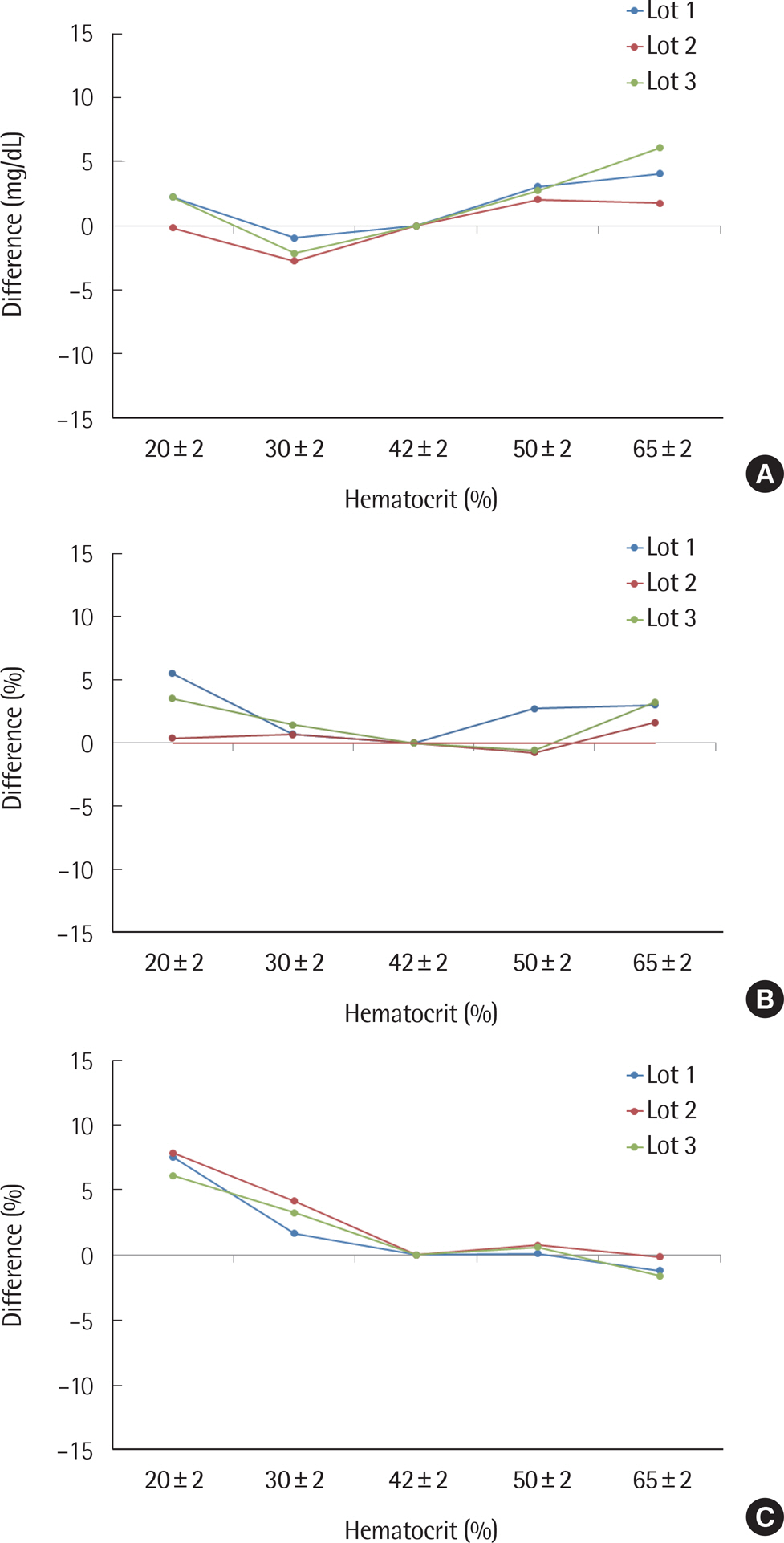
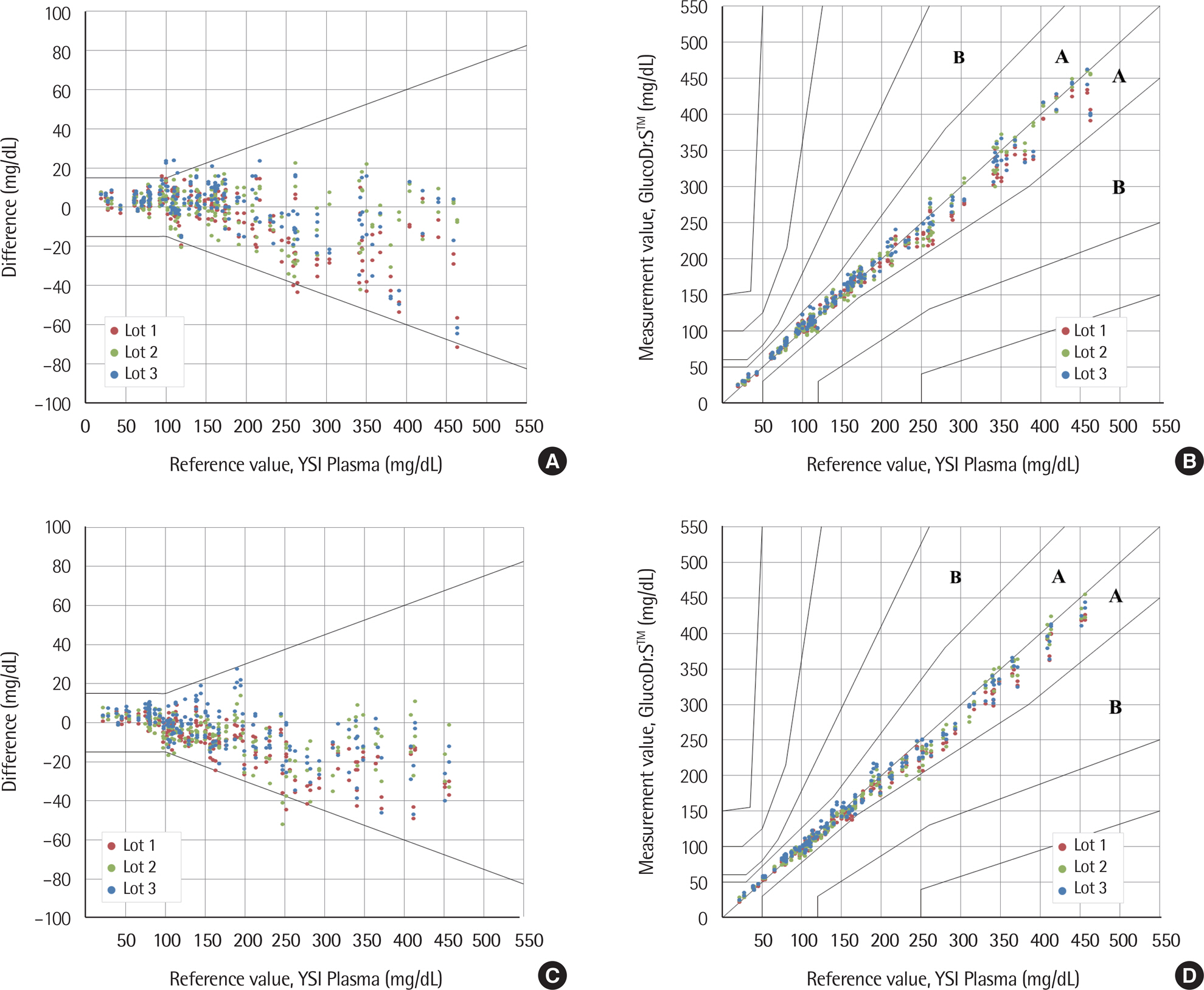
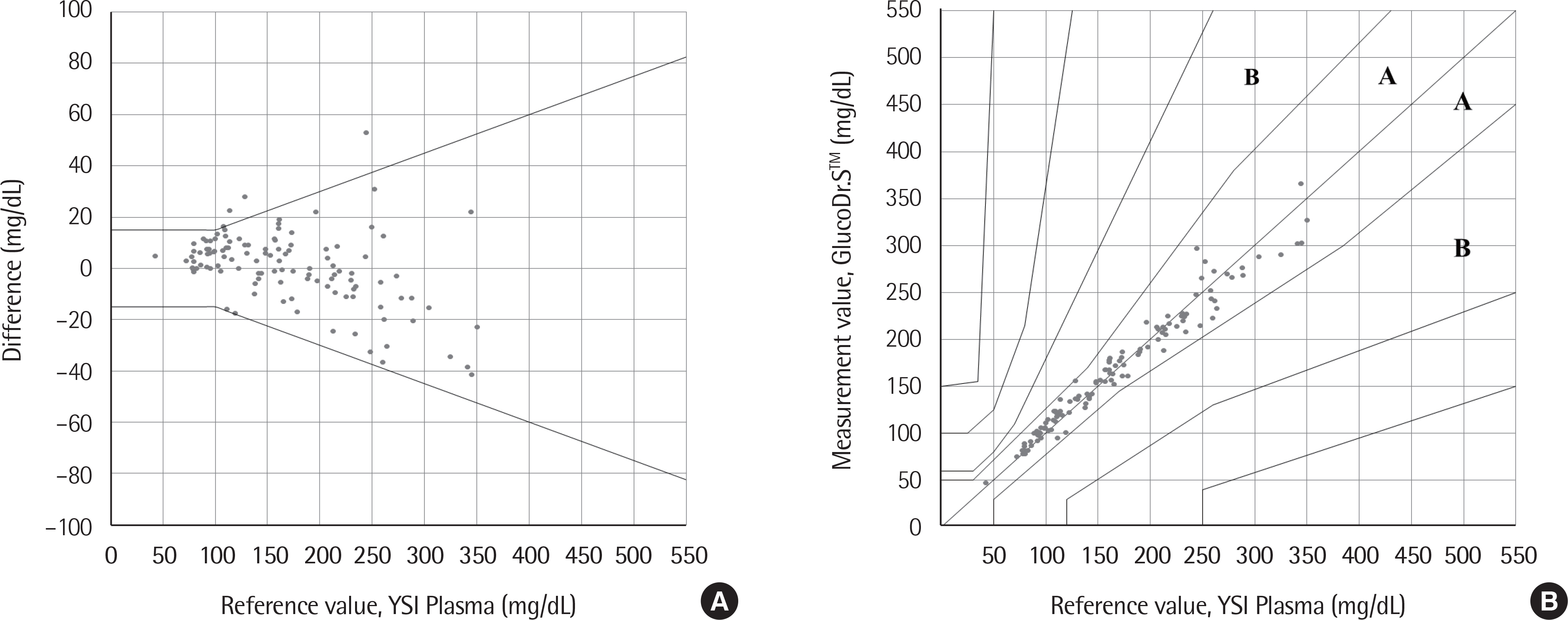
The standard deviation and coefficients of variation ranges for measurement repeatability and intermediate measurement precision conducted with 10 meters and 3 reagent lots on the same day were 2.7-3.2 mg/dL (<100 mg/dL) and 3.4-3.7% (≥100 mg/dL), respectively, and 3.7 mg/dL (<100 mg/dL) and 2.1-2.6% (≥100 mg/dL), respectively. Each coefficient of determination (R2) for linearity of the 3 reagent lots was >0.99. The influence effect of hematocrit and the 24 interference agents was not significant, except for xylose. A system accuracy test was conducted with 100 subjects taking duplicate measurements from each of the 3 reagent lots. When glucose levels were <100 mg/dL and ≥100 mg/dL, >95% of the samples were within ±15 mg/dL and within ±15% of the average measured values of the reference measurement, respectively. In Consensus Error grid analysis, all results were distributed in zone A and B. The results of the user performance evaluation using 115 lay persons were also included in the acceptance range.
CONCLUSION
The GlucoDr.Sâ„¢ showed acceptable performance according to the ISO 15197:2013 guidelines and could be a clinically useful self-testing glucometer.
MeSH Terms
Figure
Reference
-
1.Korean Diabetes Association. Diabetes fact sheet in Korea 2013. http://www.diabetes.or.kr/pro/news/admin.php?code=admin&mode=view&number=1222(UpdatedonNov2015).2.Korean Diabetes Association. Korean diabetes fact sheet 2015. http://www.diabetes.or.kr/pro/news/admin.php?code=admin&mode=view&number=1223(UpdatedonOct2016).3.Mazze RS., Shamoon H., Pasmantier R., Lucido D., Murphy J., Hartmann K, et al. Reliability of blood glucose monitoring by patients with diabetes mellitus. Am J Med. 1984. 77:211–7.
Article4.Korean Diabetes Association. Treatment guideline for diabetes. J Korean Diabetes. 2011. 12(S1):S1–S244.5.Diabetes Control and Complications Trial Research Group. Nathan DM., Genuth S., Lachin J., Cleary P., Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–86.
Article6.Mahoney J., Ellison J. Assessing the quality of glucose monitor studies: a critical evaluation of published reports. Clin Chem. 2007. 53:1122–8.
Article7.Yoo EH., Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors (Basel). 2010. 10:4558–76.
Article8.International Organization for Standardization. In vitro diagnostic test systems–Requirements for blood glucose monitoring systems for self-testing in managing diabetes mellitus. International Organization for Standardization: Geneva, Switzerland. 2003.9.International Organization for Standardization. In vitro diagnostic test systems–Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. International Organization for Standardization: Geneva, Switzerland. 2013.10.Clinical and Laboratory Standards Institute. Effects of different sample types on glucose measurements. 1st ed.POCT6. Wayne, PA: Clinical and Laboratory Standards Institute;2015.11.Clinical and Laboratory Standards Institute. Evaluation of precision of quantitative measurement procedures; approved guideline. 3rd ed.EP5-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2014.12.Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. EP6-A. Wayne, PA: Clinical and Laboratory Standards Institute. 2003.13.Clinical and Laboratory Standards Institute. Interference testing in clinical chemistry; approved guideline. 2nd ed.EP7-A2. Wayne, PA: Clinical Laboratory Standards Institute;2005.14.Clinical and Laboratory Standards Institute. Measurement procedure comparision and bias estimation using patient samples; approved guideline. 3rd ed.EP9-A3. Wayne, PA: Clinical Laboratory Standards Institute;2013.15.Freckmann G., Schmid C., Baumstark A., Rutschmann M., Haug C., Heinemann L. Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy relevant differences among ISO 15197: 2003, ISO 15197: 2013, and current FDA recommendations. J Diabetes Sci Technol. 2015. 9:885–94.16.Ministry of Food and Drug Safety of Korea. Evaluation of self-testing glucometer according to ISO 15197: 2013 guidelines. http://nifds.go.kr/nifds/03_info/sub_02.jsp?mode=view&board_no=167&article_no=9057. (Updated on Jul 2016).17.Ko DH., Lim S., Song SH., Choi SH., Park YJ., Park KU, et al. Performance evaluation of the GlucoDr plus glucometer. Diabetes Technol Ther. 2010. 12:307–12.
Article18.Food and Drug Administration. Self-monitoring blood glucose test systems for over-the-counter use—draft guidance for industry and food and drug administration staff. Food and Drug Administration. 2014.19.Clinical and Laboratory Standards Institute. Interference testing in clinical chemistry; approved guideline. 2nd ed.EP7-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2010.20.MacRury S., Srinivasan A., Mahoney JJ. Performance of a new meter designed for assisted monitoring of blood glucose and point-of-care testing. J Diabetes Sci Technol. 2013. 7:. 389–98.
Article21.Medicare, Medicaid and CLIA programs; regulations implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA)—HCFA. Final rule with comment period. Fed Regist. 1992. 57:7002–186.22.Cryer PE., Davis SN., Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003. 26:1902–12.
Article23.Lacher DA., Hughes JP., Carroll MD. Biological variation of laboratory analytes based on the 1999-2002 National Health and Nutrition Examination Survey: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. 2010.24.Kim YB., Seo JY., Lee SY., Park HD. Performance evaluation of glucometer Barozen H based on ISO 15197 standards. Lab Med Online. 2015. 5:6–14.
Article25.Kuo CY., Hsu CT., Ho CS., Su TE., Wu MH., Wang CJ. Accuracy and precision evaluation of seven self-monitoring blood glucose systems. Diabetes Technol Ther. 2011. 13:596–600.
Article26.An D., Chung HJ., Lee HW., Lee W., Chun S., Min WK. Analytical performance evaluation of glucose monitoring system following ISO15197. Korean J Lab Med. 2009. 29:423–9.
Article27.Schifman RB., Howanitz PJ., Souers RJ. Point-of-care glucose critical values: a Q-probes study involving 50 health care facilities and 2,349 critical results. Arch Pathol Lab Med. 2016. 140:119–24.
Article28.Burmeister JJ., Arnold MA. Accuracy of the YSI stat plus analyzer for glucose and lactate. Analytical Letters. 1995. 28:581–92.
Article29.Tack C., Pohlmeier H., Behnke T., Schmid V., Grenningloh M., Forst T, et al. Accuracy evaluation of fve blood glucose monitoring systems obtained from the pharmacy: a European multicenter study with 453 subjects. Diabetes Technol Ther. 2012. 14:330–7.30.Pfützner A., Mitri M., Musholt PB., Sachsenheimer D., Borchert M., Yap A, et al. Clinical assessment of the accuracy of blood glucose measurement devices. Current Med Res Opin. 2012. 28:525–31.
Article31.Rajendran R., Rayman G. Point-of-care blood glucose testing for diabetes care in hospitalized patients: an evidence-based review. J Diabetes Sci Technol. 2014. 8:1081–90.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analytic Performance Evaluation of Blood Monitoring System G400 according to ISO 15197:2013
- Performance Evaluation of B. Braun Omnitest 5 Blood Glucose Monitoring System for Self-Monitoring of Blood Glucose
- Performance Evaluation of BAROZEN H, a Networking Blood Glucose Monitoring System for Medical Institutions
- Accuracy of Capillary Blood Glucose Test When Fasting in Diabetes Patients or General Population: Performance Evaluation of G300 Based on ISO 15197:2013 Standards
- Accuracy Evaluation of GlucoLAB Auto-coding and Finetest Lite Glucose Monitoring Systems Following ISO 15197:2013