J Breast Cancer.
2016 Sep;19(3):242-251. 10.4048/jbc.2016.19.3.242.
Expression of Programmed Death Receptor Ligand 1 with High Tumor-Infiltrating Lymphocytes Is Associated with Better Prognosis in Breast Cancer
- Affiliations
-
- 1Department of Hemato-Oncology, Internal Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 2Department of Pathology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea. c84103@schmc.ac.kr
- 3Department of Surgery, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 2413948
- DOI: http://doi.org/10.4048/jbc.2016.19.3.242
Abstract
- PURPOSE
The interaction of programmed death receptor 1 (PD-1) and its ligand, programmed death receptor ligand 1 (PD-L1), negatively regulates immune responses. This study aimed to clarify PD-L1 expression levels in breast cancer through immunohistochemistry (IHC) and to evaluate associations between these findings and clinicopathologic variables, including prognosis.
METHODS
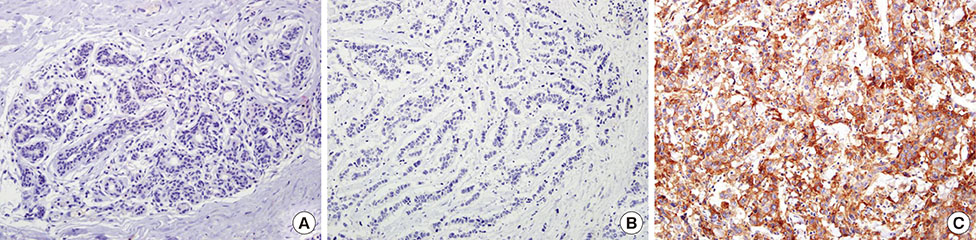
PD-L1 expression was analyzed using IHC on tissue microarrays of 465 invasive breast carcinomas.
RESULTS
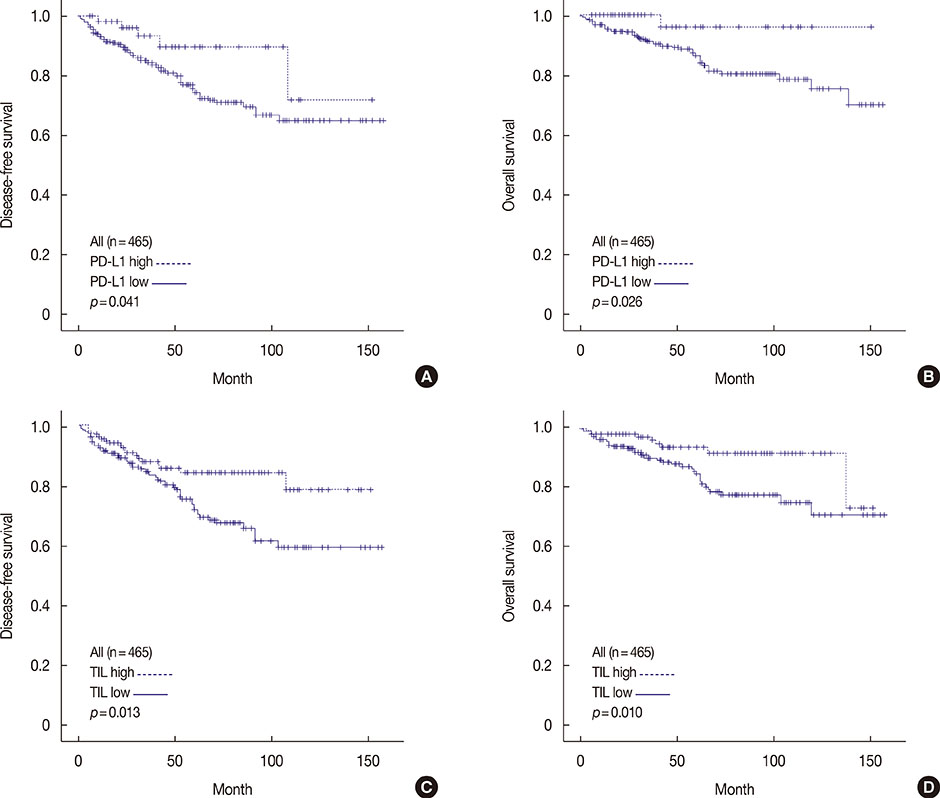
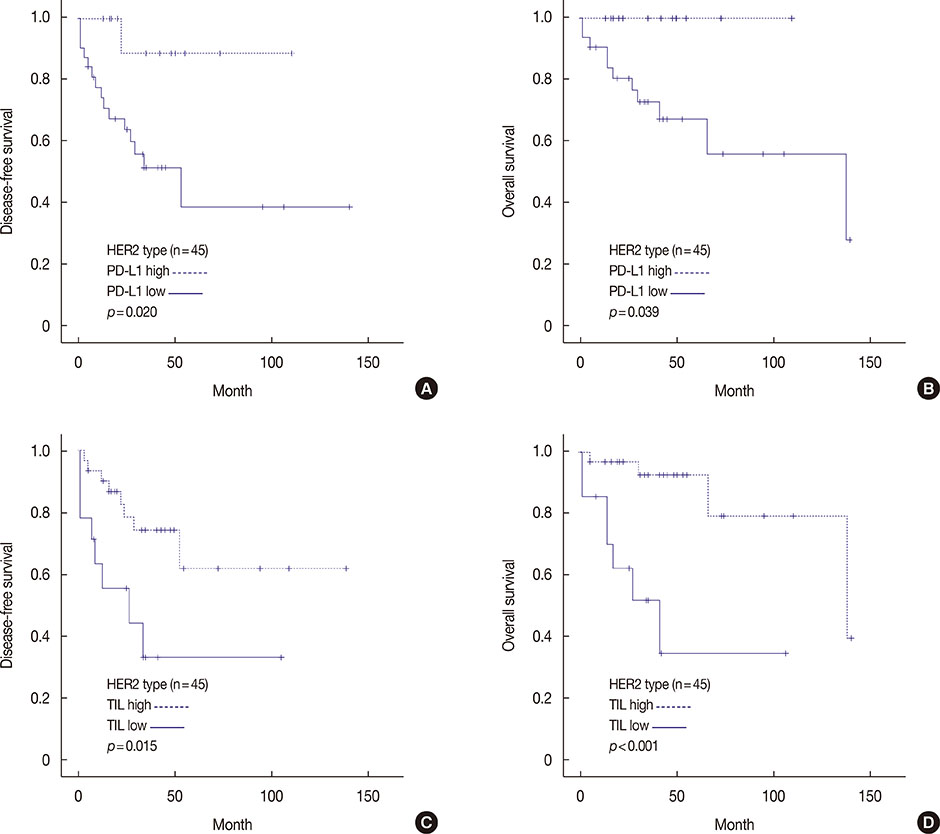
High PD-L1 expression was demonstrated in 63 of 465 tumors (13.5%). High PD-L1 expression was significantly associated with high histologic grade (p<0.001), negative lymph nodes (p=0.011), early pathologic stage (p=0.025), high tumor-infiltrating lymphocyte (TIL) (p<0.001) counts, negative estrogen receptor (p<0.001) and progesterone receptor (p=0.002) expression, positive human epidermal growth factor receptor 2 (HER2) (p=0.003), cytokeratin 5/6 (p=0.011), epidermal growth factor receptor (p<0.001), and p53 (p<0.001) expression, and high Ki-67 proliferating index (p<0.001). Based on intrinsic subtypes, high PD-L1 expression and high TIL counts were significantly associated with the HER2 and triple-negative basal type (p<0.001). PD-L1 expression was significantly associated with better disease-free survival (DFS) (p=0.041) and overall survival (OS) (p=0.026) in the univariate analysis, but not in the multivariate analysis. Higher TIL levels was an independent prognostic factor for decreased disease progression (hazard ratio [HR], 2.389; 95% confidence interval [CI], 1.284-4.445; p=0.006) and overall death (HR, 3.666; 95% CI, 1.561-8.607; p=0.003).
CONCLUSION
PD-L1 protein expression in breast cancer is associated with better DFS and OS, but is not an independent prognostic factor. High PD-L1 expression was significantly associated with high TIL levels. This finding has important implications for antibody therapies targeting the PD-1/PD-L1 signaling mechanism in breast cancer.
MeSH Terms
-
Breast Neoplasms*
Breast*
Disease Progression
Disease-Free Survival
Estrogens
Humans
Immunohistochemistry
Keratins
Lymph Nodes
Lymphocytes, Tumor-Infiltrating*
Multivariate Analysis
Prognosis*
Receptor, Epidermal Growth Factor
Receptors, Progesterone
Estrogens
Keratins
Receptor, Epidermal Growth Factor
Receptors, Progesterone
Figure
Cited by 2 articles
-
Tumor-Associated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer
Hasong Jeong, Ilseon Hwang, Sun Hee Kang, Hyeong Chan Shin, Sun Young Kwon
J Breast Cancer. 2019;22(1):38-51. doi: 10.4048/jbc.2019.22.e5.T-Cell Immunoglobulin Mucin 3 Expression on Tumor Infiltrating Lymphocytes as a Positive Prognosticator in Triple-Negative Breast Cancer
Kyung Do Byun, Hyo Jun Hwang, Ki Jae Park, Min Chan Kim, Se Heon Cho, Mi Ha Ju, Jin Hwa Lee, Jin Sook Jeong
J Breast Cancer. 2018;21(4):406-414. doi: 10.4048/jbc.2018.21.e61.
Reference
-
1. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443–2454.
Article2. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008; 26:677–704.
Article3. Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011; 241:180–205.
Article4. Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014; 94:107–116.
Article5. Phillips T, Simmons P, Inzunza HD, Cogswell J, Novotny J Jr, Taylor C, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015; 23:541–549.
Article6. Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014; 23:2965–2970.
Article7. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012; 4:127ra37.
Article8. Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013; 49:2233–2242.
Article9. Eto S, Yoshikawa K, Nishi M, Higashijima J, Tokunaga T, Nakao T, et al. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer. 2016; 19:466–471.
Article10. Oliveira-Costa JP, de Carvalho AF, da Silveira da GG, Amaya P, Wu Y, Park KJ, et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget. 2015; 6:20902–20920.
Article11. Shin SJ, Jeon YK, Kim PJ, Cho YM, Koh J, Chung DH, et al. Clinicopathologic analysis of PD-L1 and PD-L2 expression in renal cell carcinoma: association with oncogenic proteins status. Ann Surg Oncol. 2016; 23:694–702.
Article12. Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014; 146:15–24.
Article13. Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015; 3:326–332.
Article14. Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016; 47:78–84.
Article15. Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016; 47:52–63.
Article16. Cimino-Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncology (Williston Park). 2015; 29:375–385.17. Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, Liu B, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015; 26:1488–1493.
Article18. Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006; 8:190–198.
Article19. Ghebeh H, Tulbah A, Mohammed S, Elkum N, Bin Amer SM, Al-Tweigeri T, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007; 121:751–758.
Article20. Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015; 6:5449–5464.
Article21. Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014; 20:2773–2782.
Article22. Jang SH, Lee JE, Oh MH, Lee JH, Cho HD, Kim KJ, et al. High EZH2 protein expression is associated with poor overall survival in patients with luminal a breast cancer. J Breast Cancer. 2016; 19:53–60.
Article23. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015; 26:259–271.
Article24. Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014; 2:361–370.
Article25. Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One. 2014; 9:e88557.
Article26. Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010; 116:1757–1766.
Article27. Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011; 28:682–688.
Article28. Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and survival in solid tumors: a meta-analysis. PLoS One. 2015; 10:e0131403.
Article29. Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013; 1:54–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical expression of programmed death-ligand 1 and CD8 in glioblastomas
- T-Cell Immunoglobulin Mucin 3 Expression on Tumor Infiltrating Lymphocytes as a Positive Prognosticator in Triple-Negative Breast Cancer
- Prognostic Role and Clinical Association of Tumor-Infiltrating Lymphocyte, Programmed Death Ligand-1 Expression with Neutrophil-Lymphocyte Ratio in Locally Advanced Triple-Negative Breast Cancer
- Lymphocyte-Activation Gene-3 Expression and Prognostic Value in Neoadjuvant-Treated Triple-Negative Breast Cancer
- Longitudinal Intravital Imaging of Tumor-Infiltrating Lymphocyte Motility in Breast Cancer Models