J Breast Cancer.
2016 Sep;19(3):231-241. 10.4048/jbc.2016.19.3.231.
Cell-in-Cell Death Is Not Restricted by Caspase-3 Deficiency in MCF-7 Cells
- Affiliations
-
- 1The State Key Clinical Specialty in Allergy, the Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China. taoailin@gzhmu.edu.cn
- 2Guangdong Provincial Key Laboratory of Allergy & Clinical Immunology, Guangzhou Medical University, Guangzhou, China.
- 3The State Key Laboratory of Respiratory Disease, Guangzhou Medical University, Guangzhou, China.
- 4Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China.
- KMID: 2413947
- DOI: http://doi.org/10.4048/jbc.2016.19.3.231
Abstract
- PURPOSE
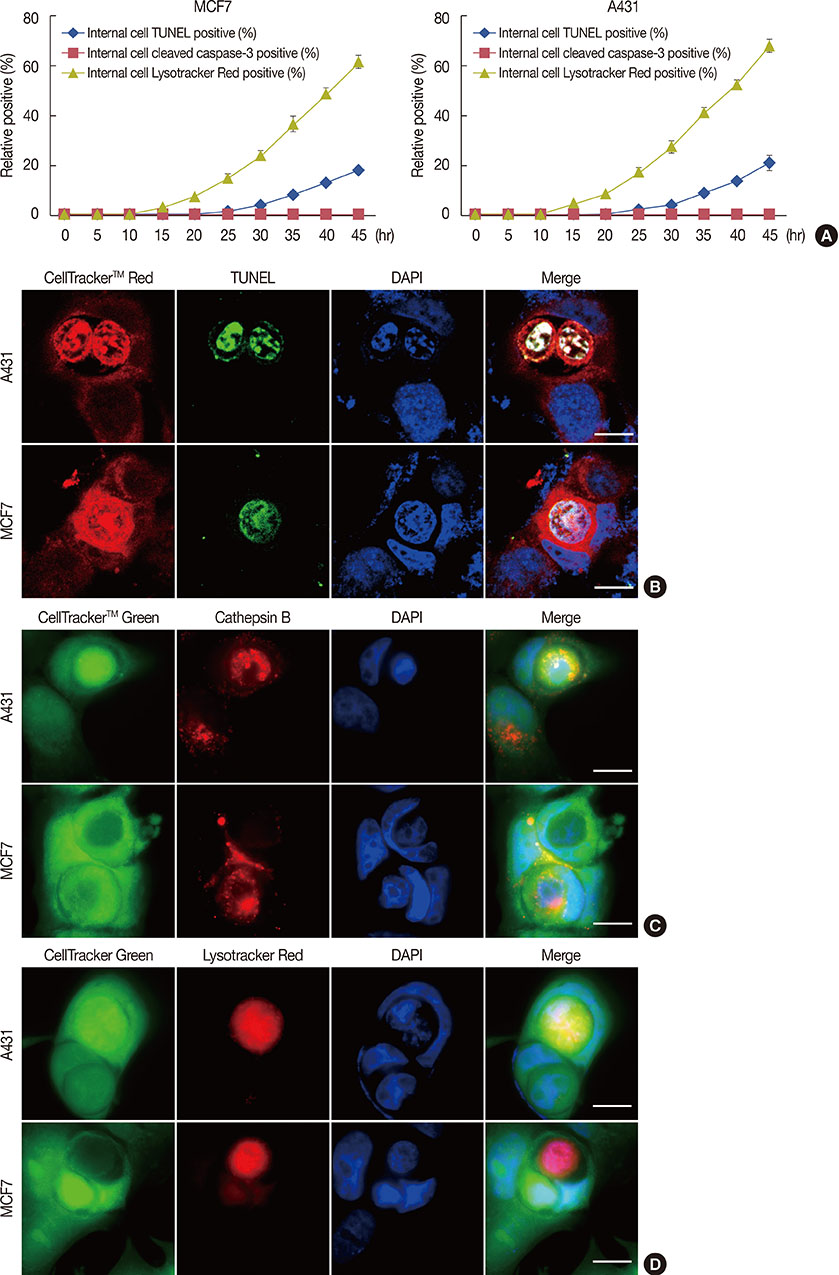
Cell-in-cell structures are created by one living cell entering another homotypic or heterotypic living cell, which usually leads to the death of the internalized cell, specifically through caspase-dependent cell death (emperitosis) or lysosome-dependent cell death (entosis). Although entosis has attracted great attention, its occurrence is controversial, because one cell line used in its study (MCF-7) is deficient in caspase-3.
METHODS
We investigated this issue using MCF-7 and A431 cell lines, which often display cell-in-cell invasion, and have different levels of caspase-3 expression. Cell-in-cell death morphology, microstructures, and signaling pathways were compared in the two cell lines.
RESULTS
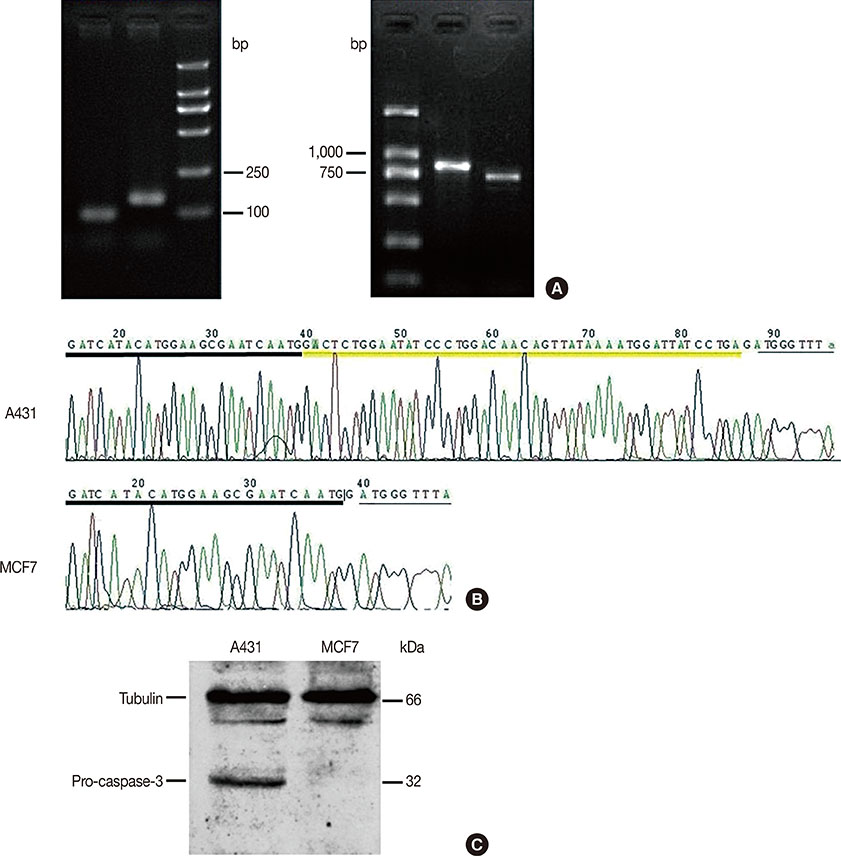
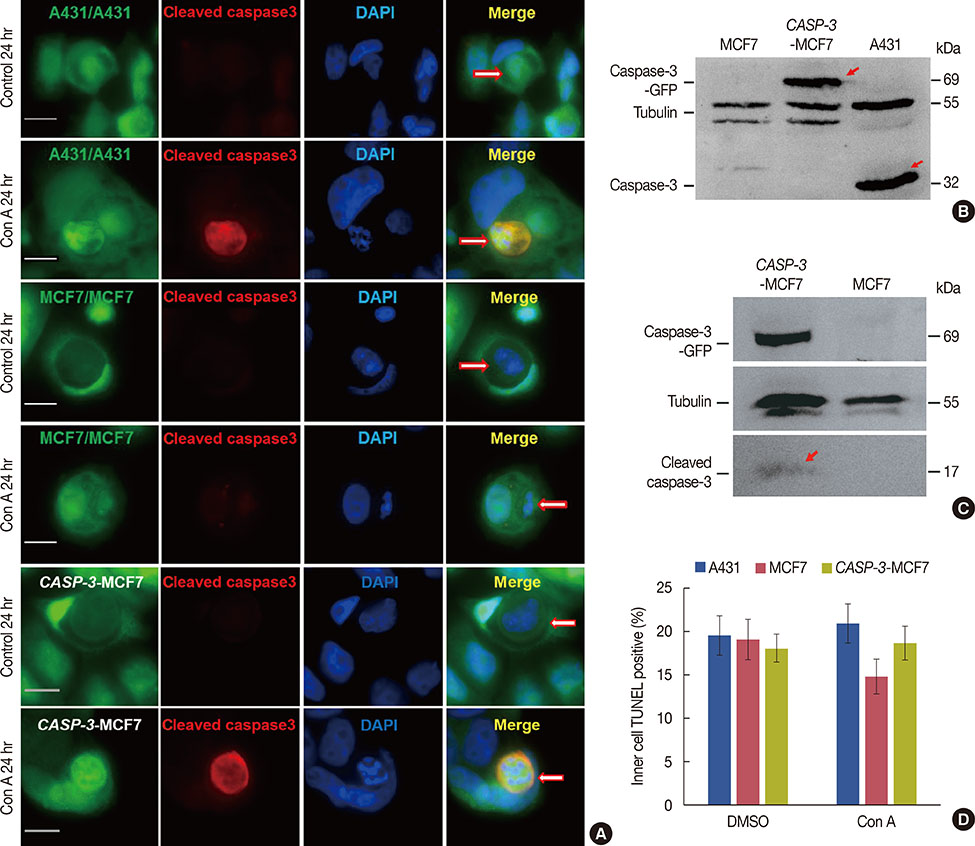
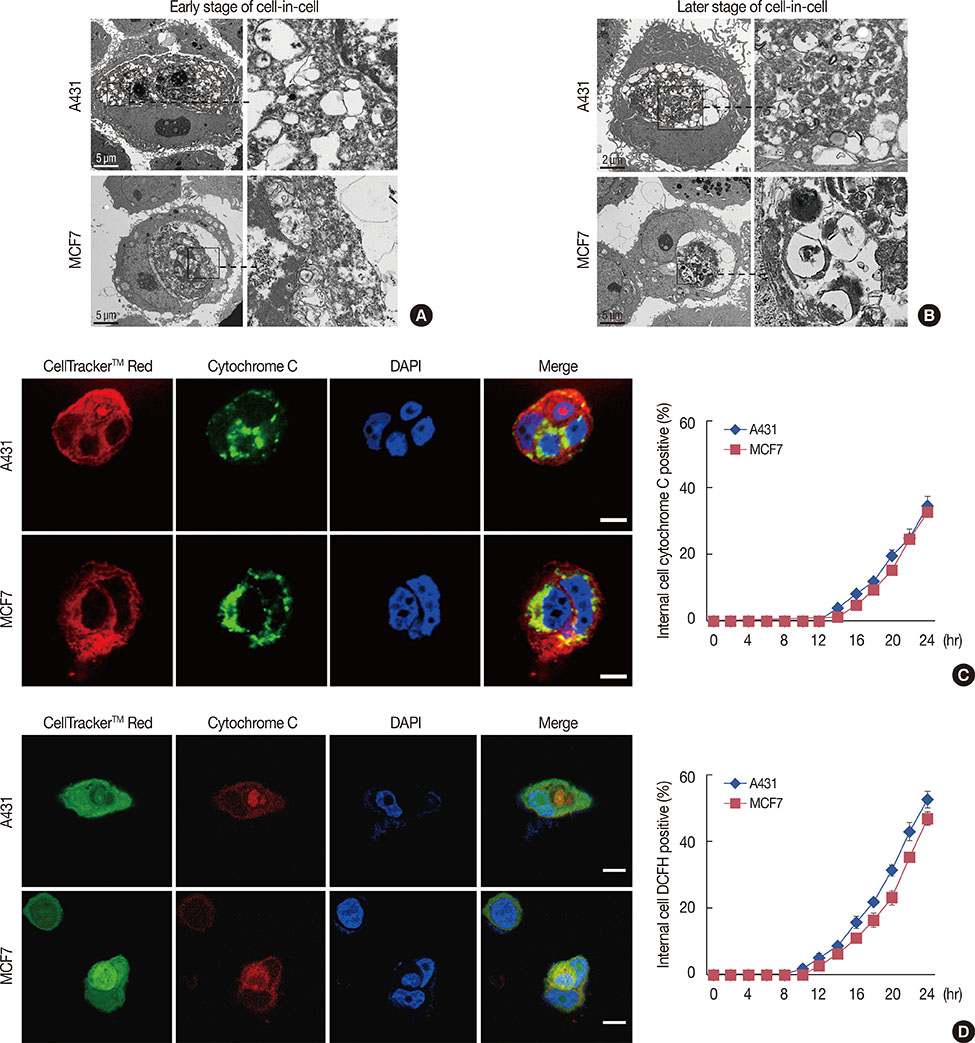
Our results confirmed that MCF-7 cells are caspase-3 deficient with a partial deletion in the CASP-3 gene. These cells underwent cell death that lacked typical apoptotic properties after staurosporine treatment, whereas caspase-3-sufficient A431 cells displayed typical apoptosis. The presence of caspase-3 was related neither to the lysosome-dependent nor to the caspase-dependent cell-in-cell death pathway. However, the existence of caspase-3 was associated with a switch from lysosome-dependent cell-in-cell death to the apoptotic cell-in-cell death pathway during entosis. Moreover, cellular hypoxia, mitochondrial swelling, release of cytochrome C, and autophagy were observed in internalized cells during entosis.
CONCLUSION
The occurrence of caspase-independent entosis is not a cell-specific process. In addition, entosis actually represents a cellular self-repair system, functioning through autophagy, to degrade damaged mitochondria resulting from cellular hypoxia in cell-in-cell structures. However, sustained autophagy-associated signal activation, without reduction in cellular hypoxia, eventually leads to lysosome-dependent intracellular cell death.
Keyword
MeSH Terms
Figure
Reference
-
1. Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009; 16:3–11.
Article2. Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007; 131:966–979.
Article3. Wang S, Guo Z, Xia P, Liu T, Wang J, Li S, et al. Internalization of NK cells into tumor cells requires ezrin and leads to programmed cell-in-cell death. Cell Res. 2009; 19:1350–1362.
Article4. Overholtzer M, Brugge JS. The cell biology of cell-in-cell structures. Nat Rev Mol Cell Biol. 2008; 9:796–809.
Article5. Wang S, He MF, Chen YH, Wang MY, Yu XM, Bai J, et al. Rapid reuptake of granzyme B leads to emperitosis: an apoptotic cell-in-cell death of immune killer cells inside tumor cells. Cell Death Dis. 2013; 4:e856.
Article6. Fais S. Cannibalism: a way to feed on metastatic tumors. Cancer Lett. 2007; 258:155–164.
Article7. Benseler V, Warren A, Vo M, Holz LE, Tay SS, Le Couteur DG, et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci U S A. 2011; 108:16735–16740.
Article8. Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol. 2011; 13:1335–1343.
Article9. Chen YH, Wang S, He MF, Wang Y, Zhao H, Zhu HY, et al. Prevalence of heterotypic tumor/immune cell-in-cell structure in vitro and in vivo leading to formation of aneuploidy. PLoS One. 2013; 8:e59418.
Article10. Jänicke RU. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res Treat. 2009; 117:219–221.
Article11. Feng FF, Zhang DR, Tian KL, Lou HY, Qi XL, Wang YC, et al. Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by oridonin nanosuspension. Drug Deliv. 2011; 18:265–271.
Article12. Chen JS, Konopleva M, Andreeff M, Multani AS, Pathak S, Mehta K. Drug-resistant breast carcinoma (MCF-7) cells are paradoxically sensitive to apoptosis. J Cell Physiol. 2004; 200:223–234.
Article13. Jänicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998; 273:9357–9360.
Article14. Langford MP, Chen D, Gosslee J, Misra RP, Redens TB, Texada DE. Intracameral toxicity of bacterial components muramyl dipeptide and staurosporine: ciliary cyst formation, epithelial cell apoptosis and necrosis. Cutan Ocul Toxicol. 2006; 25:85–101.
Article15. Li M, Khambu B, Zhang H, Kang JH, Chen X, Chen D, et al. Suppression of lysosome function induces autophagy via a feedback down-regulation of MTOR complex 1 (MTORC1) activity. J Biol Chem. 2013; 288:35769–35780.
Article16. Lugini L, Matarrese P, Tinari A, Lozupone F, Federici C, Iessi E, et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006; 66:3629–3638.
Article17. Rausch V, Liu L, Apel A, Rettig T, Gladkich J, Labsch S, et al. Autophagy mediates survival of pancreatic tumour-initiating cells in a hypoxic microenvironment. J Pathol. 2012; 227:325–335.
Article18. Wojtkowiak JW, Gillies RJ. Autophagy on acid. Autophagy. 2012; 8:1688–1689.
Article19. Garg AD, Dudek AM, Ferreira GB, Verfaillie T, Vandenabeele P, Krysko DV, et al. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy. 2013; 9:1292–1307.
Article20. Macintosh RL, Ryan KM. Autophagy in tumour cell death. Semin Cancer Biol. 2013; 23:344–351.
Article21. Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012; 19:87–95.
Article22. Minina EA, Bozhkov PV, Hofius D. Autophagy as initiator or executioner of cell death. Trends Plant Sci. 2014; 19:692–697.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptotic Effects of 6-Gingerol in Human Breast Cancer Cells
- Effects of Triterpenoids from Luvunga scandens on Cytotoxic, Cell Cycle Arrest and Gene Expressions in MCF-7 Cells
- Cell Death Induction Mechanism of Non-small Cell Lung Cancer Cell Line, NCI-H1703 by Docetaxel
- Caspase is Regulated by ROS in CT Induced Neuronal Cell Death
- Cell Death and Immunity