J Breast Cancer.
2018 Jun;21(2):222-226. 10.4048/jbc.2018.21.2.222.
Verification of a Western Nomogram for Predicting Oncotype DXâ„¢ Recurrence Scores in Korean Patients with Breast Cancer
- Affiliations
-
- 1Department of Surgery, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, Jinju, Korea.
- 2Division of Breast and Endocrine Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. paojlus@hanmail.net
- KMID: 2413942
- DOI: http://doi.org/10.4048/jbc.2018.21.2.222
Abstract
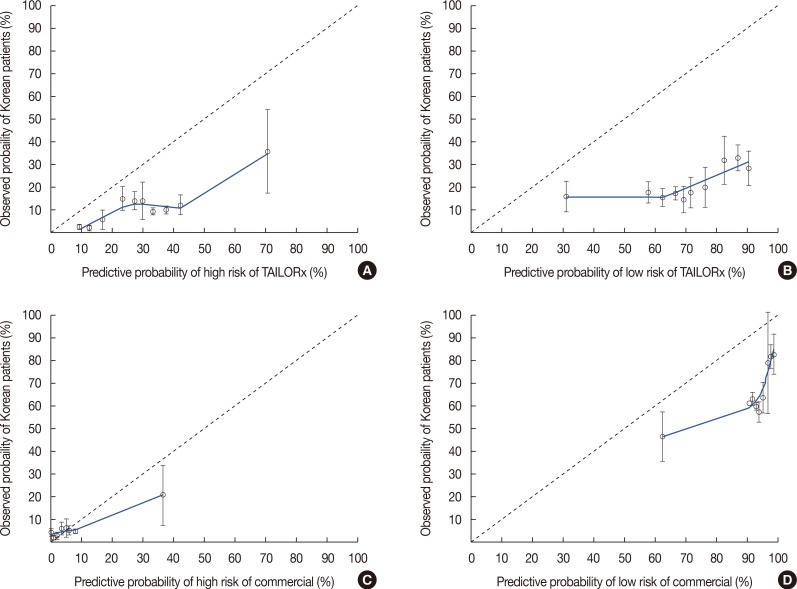
- A recent study conducted at the University of Tennessee Medical Center using a large dataset from the National Cancer Database (NCDB) reported the use of nomograms for predicting Oncotype DXâ„¢ (ODX) scores with clinicopathologic data. We reviewed the data of 218 patients who underwent the ODX test at a single institution in Korea to confirm that nomograms can accurately predict ODX score groups using our data, which differ from those of the NCDB in terms of ethnicity. The concordance index (c-index) of nomograms was much lower than that of the University of Tennessee Medical Center for high- and low-risk groups of commercial ODX and Trial Assigning Individualized Options for Treatment values. Although the nomogram for predicting ODX scores was based on a large dataset, it could not be generalized to patients in Asia. Further studies using large datasets of patients from different ethnicities should be performed to develop a nomogram applicable to patients worldwide.
Keyword
MeSH Terms
Figure
Reference
-
1. Halpern N, Sonnenblick A, Uziely B, Divinsky L, Goldberg Y, Hamburger T, et al. Oncotype Dx recurrence score among BRCA1/2 germline mutation carriers with hormone receptors positive breast cancer. Int J Cancer. 2017; 140:2145–2149. PMID: 28120435.
Article2. NSABP study confirms Oncotype DX predicts chemotherapy benefit in breast cancer patients. Oncology (Williston Park). 2006; 20:789–790. PMID: 16841801.3. Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006; 24:3726–3734. PMID: 16720680.
Article4. Sparano JA. TAILORx: trial assigning individualized options for treatment (Rx). Clin Breast Cancer. 2006; 7:347–350. PMID: 17092406.
Article5. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. NCCN guidelines insights breast cancer, version 1.2016. J Natl Compr Canc Netw. 2015; 13:1475–1485. PMID: 26656517.6. Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016; 34:1134–1150. PMID: 26858339.
Article7. Orucevic A, Heidel RE, Bell JL. Utilization and impact of 21-gene recurrence score assay for breast cancer in clinical practice across the United States: lessons learned from the 2010 to 2012 National Cancer Data Base analysis. Breast Cancer Res Treat. 2016; 157:427–435. PMID: 27206678.
Article8. Albanell J, Svedman C, Gligorov J, Holt SD, Bertelli G, Blohmer JU, et al. Pooled analysis of prospective European studies assessing the impact of using the 21-gene Recurrence Score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer. Eur J Cancer. 2016; 66:104–113. PMID: 27544930.
Article9. Orucevic A, Bell JL, McNabb AP, Heidel RE. Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat. 2017; 163:51–61. PMID: 28243897.
Article10. Lee MH, Han W, Lee JE, Kim KS, Park H, Kim J, et al. The clinical impact of 21-gene recurrence score on treatment decisions for patients with hormone receptor-positive early breast cancer in Korea. Cancer Res Treat. 2015; 47:208–214. PMID: 25381828.
Article11. Gross domestic product. Statistics Korea. Accessed August 5th, 2017. http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_2KAAG02&vw_cd.12. GDP & personal income. Bureau of Economic Analysis. Accessed August 5th, 2017. https://www.bea.gov/iTable/index_nipa.cfm.13. Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016; 34:3308–3314. PMID: 27480155.
Article14. Enewold L, Geiger AM, Zujewski J, Harlan LC. Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat. 2015; 151:149–156. PMID: 25859924.
Article15. Roberts MC, Weinberger M, Dusetzina SB, Dinan MA, Reeder-Hayes KE, Troester MA, et al. Racial variation in adjuvant chemotherapy initiation among breast cancer patients receiving Oncotype DX testing. Breast Cancer Res Treat. 2015; 153:191–200. PMID: 26216535.
Article16. Albain KS, Barlow WE, Shak S, Hortobagyi GN, Hayes DF. The Breast Cancer Intergroup of North America. Potential biologic causes of the racial survival disparity in adjuvant trials of ER-positive breast cancer. J Clin Oncol. 2010; 28(15 Suppl):511.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Nomogram for Predicting the Oncotype DX Recurrence Score in Women with T1-3N0-1miM0 Hormone Receptor‒Positive, Human Epidermal Growth Factor 2 (HER2)‒Negative Breast Cancer
- A Novel Prognostic Nomogram for Predicting Risks of Distant Failure in Patients with Invasive Breast Cancer Following Postoperative Adjuvant Radiotherapy
- Automated immunohistochemical assessment ability to evaluate estrogen and progesterone receptor status compared with quantitative reverse transcription-polymerase chain reaction in breast carcinoma patients
- Predictive value of chest computed tomography for axillary lymph node metastasis in patients with breast cancer A retrospective cohort study
- The Clinical Impact of 21-Gene Recurrence Score on Treatment Decisions for Patients with Hormone Receptor-Positive Early Breast Cancer in Korea