J Breast Cancer.
2018 Jun;21(2):150-157. 10.4048/jbc.2018.21.2.150.
Efficiency of Cytokine-Induced Killer Cells in Combination with Chemotherapy for Triple-Negative Breast Cancer
- Affiliations
-
- 1Department of Biotherapy, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China. rwziyi@yahoo.com
- 2National Clinical Research Center for Cancer, Tianjin, China.
- 3Key Laboratory of Cancer Prevention and Therapy, Tianjin, China.
- 4Tianjin's Clinical Research Center for Cancer, Tianjin, China.
- 5Key Laboratory of Cancer Immunology and Biotherapy, Tianjin, China.
- 6Department of Immunology, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China.
- KMID: 2413933
- DOI: http://doi.org/10.4048/jbc.2018.21.2.150
Abstract
- PURPOSE
The treatment of triple-negative breast cancer (TNBC) remains challenging, due to the absence of estrogen, progesterone, and human epidermal growth factor receptors. This study was designed to evaluate the efficiency and safety of cytokine-induced killer (CIK) cell immunotherapy, following regular chemotherapy, for patients with TNBC.
METHODS
A total of 340 patients with postmastectomy TNBC, from January 1, 2010 to June 30, 2014, were included in this retrospective study. Seventy-seven patients received CIK cell immunotherapy, following regular chemotherapy (arm 1), and 263 patients received regular chemotherapy alone (arm 2). The primary aim was overall survival (OS) and disease-free survival (DFS), and the treatment responses and adverse events were also evaluated.
RESULTS
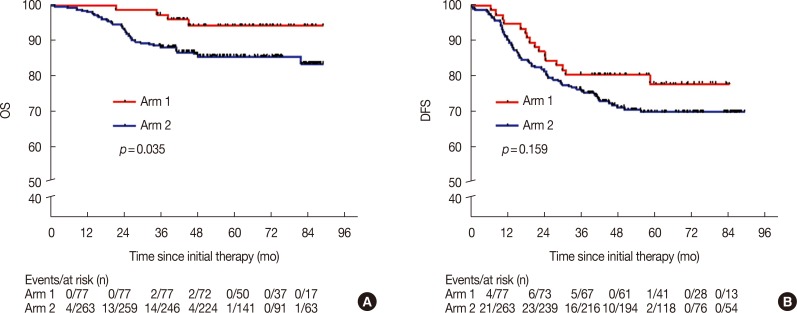
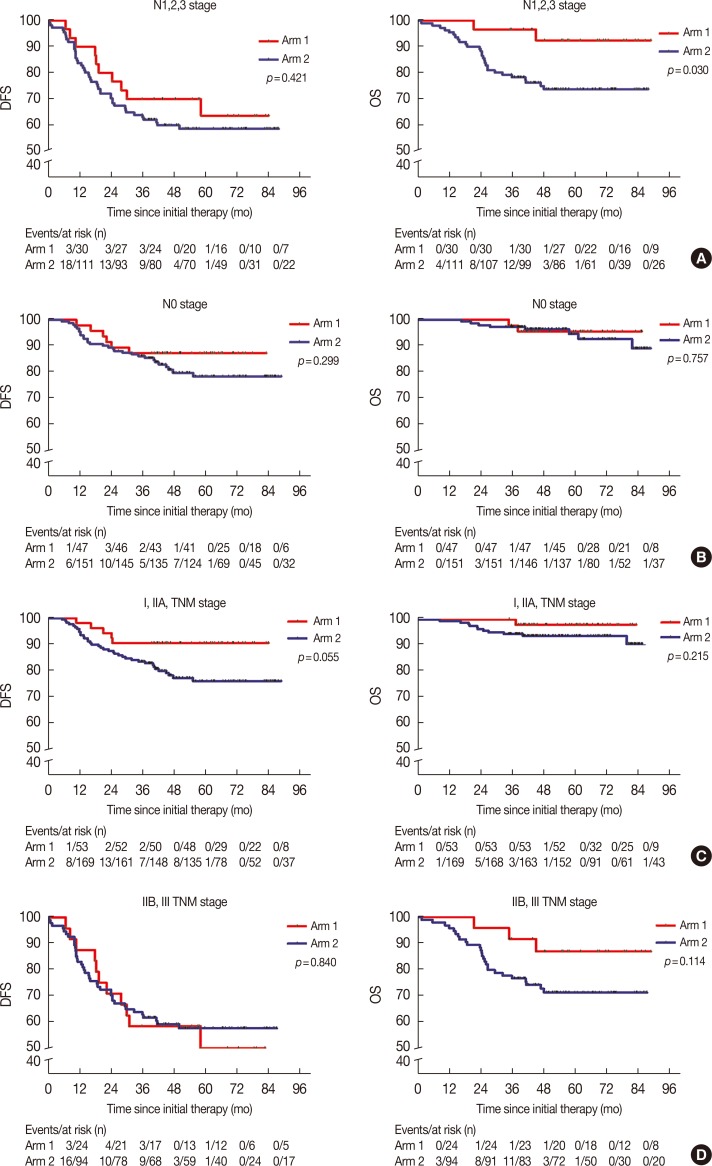
The 5-year DFS and OS rates in arm 1 were 77.9% and 94.3%, compared with 69.8% and 85.6% in arm 2, respectively (p=0.159 and p=0.035, respectively). This clearly shows that there was no statistical difference in the 5-year DFS between the two groups. Multivariate analyses of arm 1 indicated that a Karnofsky performance score (KPS) ≥90 and stage I/IIA disease were significantly associated with a prolonged DFS period (hazard ratio [HR], 0.25; 95% confidence interval [CI], 0.09-0.74; p=0.012; and HR 0.21; 95% CI, 0.06-0.82; p=0.024, respectively), but a KPS ≥90 and stage I/IIA disease were not independent prognostic factors for OS. Toxicity was mild in patients who received the CIK therapy.
CONCLUSION
The data suggested that CIK cell immunotherapy improved the efficiency of regular chemotherapy in patients with TNBC, and the side effects of CIK cell immunotherapy were mild.
Keyword
MeSH Terms
Figure
Reference
-
1. Benson JR, Jatoi I. The global breast cancer burden. Future Oncol. 2012; 8:697–702. PMID: 22764767.
Article2. Guo P, Huang ZL, Yu P, Li K. Trends in cancer mortality in China: an update. Ann Oncol. 2012; 23:2755–2762. PMID: 22492700.
Article3. Clarke M. Meta-analyses of adjuvant therapies for women with early breast cancer: the Early Breast Cancer Trialists' Collaborative Group overview. Ann Oncol. 2006; 17(Suppl 10):x59–x62. PMID: 17018753.
Article4. Mouh FZ, Mzibri ME, Slaoui M, Amrani M. Recent progress in triple negative breast cancer research. Asian Pac J Cancer Prev. 2016; 17:1595–1608. PMID: 27221827.
Article5. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13(15 Pt 1):4429–4434. PMID: 17671126.
Article6. Lara-Medina F, Pérez-Sánchez V, Saavedra-Pérez D, Blake-Cerda M, Arce C, Motola-Kuba D, et al. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011; 117:3658–3669. PMID: 21387260.7. Joensuu H, Gligorov J. Adjuvant treatments for triple-negative breast cancers. Ann Oncol. 2012; 23(Suppl 6):vi40–vi45. PMID: 23012301.
Article8. Schwentner L, Wolters R, Koretz K, Wischnewsky MB, Kreienberg R, Rottscholl R, et al. Triple-negative breast cancer: the impact of guideline-adherent adjuvant treatment on survival: a retrospective multicentre cohort study. Breast Cancer Res Treat. 2012; 132:1073–1080. PMID: 22205141.9. Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol. 2011; 137:305–310. PMID: 20407789.
Article10. Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011; 17:6287–6297. PMID: 21844012.
Article11. Yu R, Yang B, Chi X, Cai L, Liu C, Yang L, et al. Efficacy of cytokine-induced killer cell infusion as an adjuvant immunotherapy for hepatocellular carcinoma: a systematic review and meta-analysis. Drug Des Devel Ther. 2017; 11:851–864.
Article12. Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993; 21:1673–1679. PMID: 7694868.13. Schmeel FC, Schmeel LC, Gast SM, Schmidt-Wolf IG. Adoptive immunotherapy strategies with cytokine-induced killer (CIK) cells in the treatment of hematological malignancies. Int J Mol Sci. 2014; 15:14632–14648. PMID: 25196601.14. Liu L, Zhang W, Qi X, Li H, Yu J, Wei S, et al. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin Cancer Res. 2012; 18:1751–1759. PMID: 22275504.
Article15. Zhao H, Wang Y, Yu J, Wei F, Cao S, Zhang X, et al. Autologous cytokine-induced killer cells improves overall survival of metastatic colorectal cancer patients: results from a phase ii clinical trial. Clin Colorectal Cancer. 2016; 15:228–235. PMID: 27052743.
Article16. Wang Z, Liu Y, Li R, Shang Y, Zhang Y, Zhao L, et al. Autologous cytokine-induced killer cell transfusion increases overall survival in advanced pancreatic cancer. J Hematol Oncol. 2016; 9:6. PMID: 26842696.
Article17. Li R, Wang C, Liu L, Du C, Cao S, Yu J, et al. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother. 2012; 61:2125–2133. PMID: 22581306.
Article18. Liu J, Li H, Cao S, Zhang X, Yu J, Qi J, et al. Maintenance therapy with autologous cytokine-induced killer cells in patients with advanced epithelial ovarian cancer after first-line treatment. J Immunother. 2014; 37:115–122. PMID: 24509174.
Article19. Schmeel LC, Schmeel FC, Coch C, Schmidt-Wolf IG. Cytokine-induced killer (CIK) cells in cancer immunotherapy: report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol. 2015; 141:839–849. PMID: 25381063.
Article20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.
Article21. Stagg J, Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol. 2013; 5:169–181. PMID: 23634195.
Article22. Gammaitoni L, Giraudo L, Leuci V, Todorovic M, Mesiano G, Picciotto F, et al. Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features. Clin Cancer Res. 2013; 19:4347–4358. PMID: 23794732.
Article23. Sangiolo D, Mesiano G, Gammaitoni L, Leuci V, Todorovic M, Giraudo L, et al. Cytokine-induced killer cells eradicate bone and soft-tissue sarcomas. Cancer Res. 2014; 74:119–129. PMID: 24356422.
Article24. Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010; 120:1111–1124. PMID: 20234093.
Article25. Pan K, Guan XX, Li YQ, Zhao JJ, Li JJ, Qiu HJ, et al. Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res. 2014; 20:3003–3011. PMID: 24668644.
Article26. Ho AY, Gupta G, King TA, Perez CA, Patil SM, Rogers KH, et al. Favorable prognosis in patients with T1a/T1bN0 triple-negative breast cancers treated with multimodality therapy. Cancer. 2012; 118:4944–4952. PMID: 22392492.
Article27. Li H, Huang L, Liu L, Wang X, Zhang Z, Yue D, et al. Selective effect of cytokine-induced killer cells on survival of patients with early-stage melanoma. Cancer Immunol Immunother. 2017; 66:299–308. PMID: 27889798.
Article28. Li DP, Li W, Feng J, Chen K, Tao M. Adjuvant chemotherapy with sequential cytokine-induced killer (CIK) cells in stage IB non-small cell lung cancer. Oncol Res. 2015; 22:67–74. PMID: 25706393.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell-based Immunotherapy for Colorectal Cancer with Cytokine-induced Killer Cells
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- Inhibition of Human Pancreatic Tumor Growth by Cytokine-Induced Killer Cells in Nude Mouse Xenograft Model
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Hsa-miRNA-143-3p Reverses Multidrug Resistance of Triple-Negative Breast Cancer by Inhibiting the Expression of Its Target Protein Cytokine-Induced Apoptosis Inhibitor 1 In Vivo