J Breast Cancer.
2018 Jun;21(2):124-133. 10.4048/jbc.2018.21.2.124.
Lymphocyte-Activation Gene-3 Expression and Prognostic Value in Neoadjuvant-Treated Triple-Negative Breast Cancer
- Affiliations
-
- 1Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China. zqyxsci@126.com
- 2Department of Pathology, Harbin Medical University Cancer Hospital, Harbin, China.
- 3Cancer Research Institute of Heilongjiang, Harbin, China.
- KMID: 2413930
- DOI: http://doi.org/10.4048/jbc.2018.21.2.124
Abstract
- PURPOSE
In this study, we aimed to evaluate lymphocyte-activation gene-3 (LAG-3) expression and its prognostic value in neoadjuvant-treated triple-negative breast cancer (TNBC).
METHODS
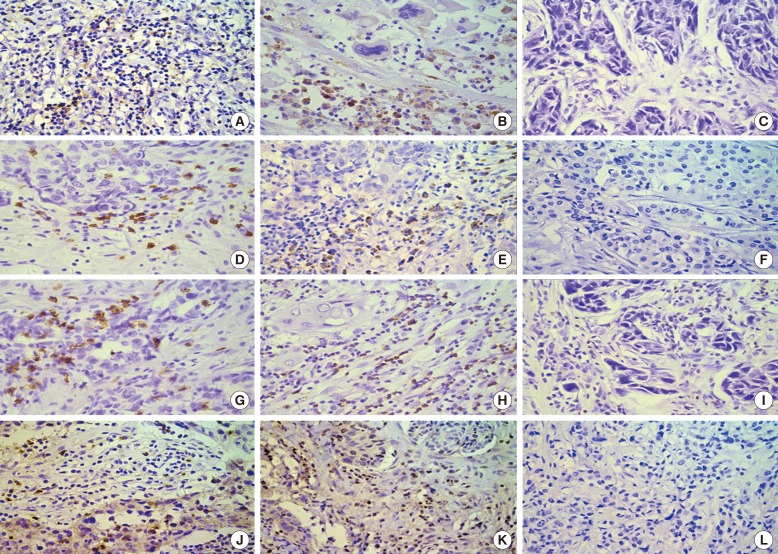
LAG-3, programmed death-1 (PD-1), programmed death ligand-1 (PD-L1), and CD8⺠tumor-infiltrating lymphocyte (TILs) levels were examined using immunohistochemistry in 148 preand 114 post-neoadjuvant chemotherapy (NACT) specimens of human TNBC tissue. Correlations between expression levels and clinicopathological features were analyzed. Prognostic values for combined detection in TNBC following NACT were evaluated.
RESULTS
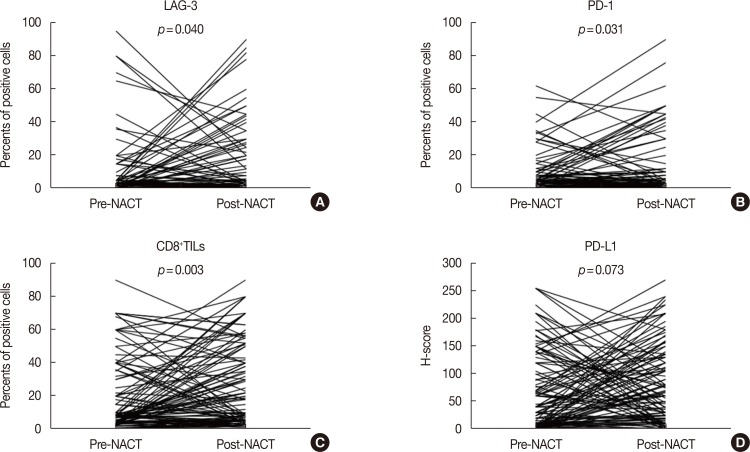
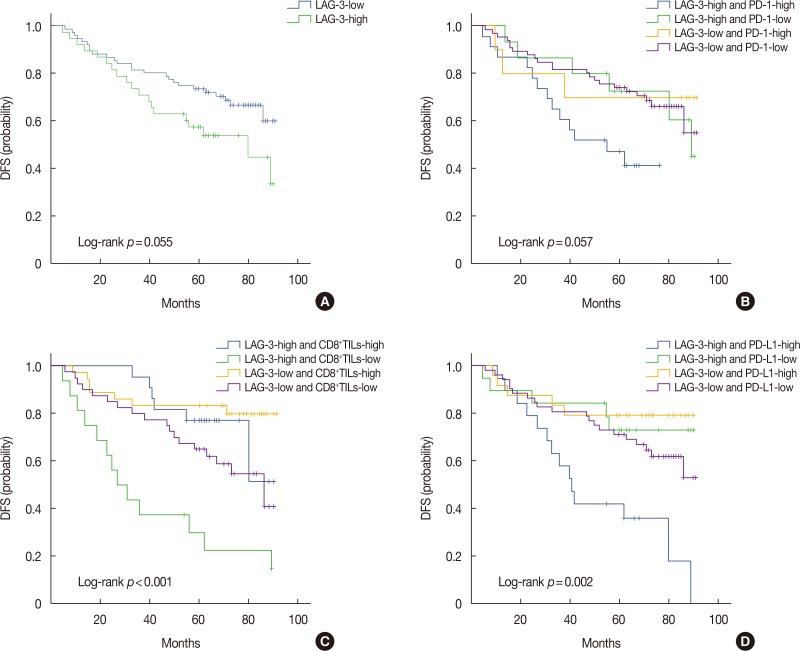
In pre-NACT specimens, LAG-3 expression showed a significant association with pathological complete response (pCR, p=0.038) and was correlated with PD-1 (p<0.001) and PD-L1 (p=0.008). In post-NACT specimens, high expression of LAG-3 showed significant effects on nodal status (p=0.023) and PD-1 (p<0.001). The expression of immune markers on TILs significantly increased following NACT. Multivariate analysis indicated that only nodal status (odds ratio [OR], 0.226; 95% confidence interval [CI], 0.079-0.644; p=0.005) and high quantities of CD8âºTILs (OR, 3.186; 95% CI, 1.314-7.721; p=0.010) are independent predictors of pCR. Nodal status (hazard ratio [HR], 2.666; 95% CI, 1.271-5.594; p=0.010), CD8âºTILs (HR, 0.313; 95% CI, 0.139-0.705; p=0.005), and the LAG-3-high/PD-L1-high group (HR, 2.829; 95% CI, 1.050-7.623; p=0.040) provided prognostic values for patients with TNBC following NACT.
CONCLUSION
CD8+TILs were sensitive predictive markers in response to NACT. High expression of LAG-3 in residual tissues, especially in combination with PD-L1, was associated with poor prognosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007; 109:25–32. PMID: 17146782.
Article2. Caudle AS, Yu TK, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012; 14:R83. PMID: 22621334.
Article3. Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014; 32:2959–2966. PMID: 25071121.4. Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015; 33:983–991. PMID: 25534375.
Article5. Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M, et al. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015; 13:202. PMID: 26300242.
Article6. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012; 366:2455–2465. PMID: 22658128.
Article7. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443–2454. PMID: 22658127.
Article8. Turnis ME, Andrews LP, Vignali DA. Inhibitory receptors as targets for cancer immunotherapy. Eur J Immunol. 2015; 45:1892–1905. PMID: 26018646.
Article9. Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010; 107:7875–7880. PMID: 20385810.10. Bottai G, Raschioni C, Losurdo A, Di Tommaso L, Tinterri C, Torrisi R, et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res. 2016; 18:121. PMID: 27912781.
Article11. Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res. 2015; 21:3969–3976. PMID: 25944800.
Article12. Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013; 139:667–676. PMID: 23756627.
Article13. Song IH, Heo SH, Bang WS, Park HS, Park IA, Kim YA, et al. Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple-negative breast cancer. Cancer Res Treat. 2017; 49:399–407. PMID: 27488875.
Article14. Li Z, Dong P, Ren M, Song Y, Qian X, Yang Y, et al. PD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J Cancer. 2016; 7:784–793. PMID: 27162536.
Article15. Waugh KA, Leach SM, Moore BL, Bruno TC, Buhrman JD, Slansky JE. Molecular profile of tumor-specific CD8+ T cell hypofunction in a transplantable murine cancer model. J Immunol. 2016; 197:1477–1488. PMID: 27371726.
Article16. Cappello P, Triebel F, Iezzi M, Caorsi C, Quaglino E, Lollini PL, et al. LAG-3 enables DNA vaccination to persistently prevent mammary carcinogenesis in HER-2/neu transgenic BALB/c mice. Cancer Res. 2003; 63:2518–2525. PMID: 12750275.17. Li FJ, Zhang Y, Jin GX, Yao L, Wu DQ. Expression of LAG-3 is coincident with the impaired effector function of HBV-specific CD8(+) T cell in HCC patients. Immunol Lett. 2013; 150:116–122. PMID: 23261718.
Article18. Foy SP, Sennino B, dela Cruz T, Cote JJ, Gordon EJ, Kemp F, et al. Poxvirus-based active immunotherapy with PD-1 and LAG-3 dual immune checkpoint inhibition overcomes compensatory immune regulation, yielding complete tumor regression in mice. PLoS One. 2016; 11:e0150084. PMID: 26910562.
Article19. He Y, Yu H, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, et al. LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol. 2017; 12:814–823. PMID: 28132868.20. Mesnage SJ, Auguste A, Genestie C, Dunant A, Pain E, Drusch F, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann Oncol. 2017; 28:651–657. PMID: 27864219.
Article21. Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-kappaB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015; 75:5034–5045. PMID: 26573793.22. Teng F, Meng X, Kong L, Mu D, Zhu H, Liu S, et al. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. 2015; 166:721–732.e1. PMID: 26209749.
Article23. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014; 124:687–695. PMID: 24382348.
Article24. Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013; 1:365–372. PMID: 24563870.
Article25. Son CH, Bae JH, Shin DY, Lee HR, Choi YJ, Jo WS, et al. CTLA-4 blockade enhances antitumor immunity of intratumoral injection of immature dendritic cells into irradiated tumor in a mouse colon cancer model. J Immunother. 2014; 37:1–7. PMID: 24316550.
Article26. Al-Saleh K, Abd El-Aziz N, Ali A, Abozeed W, Abd El-Warith A, Ibraheem A, et al. Predictive and prognostic significance of CD8(+) tumor-infiltrating lymphocytes in patients with luminal B/HER 2 negative breast cancer treated with neoadjuvant chemotherapy. Oncol Lett. 2017; 14:337–344. PMID: 28693173.
Article27. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013; 369:134–144. PMID: 23724846.
Article28. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012; 72:917–927. PMID: 22186141.
Article29. Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3: potential mechanisms of action. Nat Rev Immunol. 2015; 15:45–56. PMID: 25534622.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- Differences in prognosis by p53 expression after neoadjuvant chemotherapy in triple-negative breast cancer
- Molecular Classification of Triple-Negative Breast Cancer
- Prognostic Role and Clinical Association of Tumor-Infiltrating Lymphocyte, Programmed Death Ligand-1 Expression with Neutrophil-Lymphocyte Ratio in Locally Advanced Triple-Negative Breast Cancer
- Gene Regulatory Network Analysis for Triple-Negative Breast Neoplasms by Using Gene Expression Data