Korean J Radiol.
2018 Aug;19(4):758-766. 10.3348/kjr.2018.19.4.758.
Evaluation of Salivary Gland Function Using Diffusion-Weighted Magnetic Resonance Imaging for Follow-Up of Radiation-Induced Xerostomia
- Affiliations
-
- 1Department of Radiology, Shanghai Proton and Heavy Ion Center, Shanghai 201321, China.
- 2Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, China.
- 3Department of Radiology, Fudan University Shanghai Cancer Center, Shanghai 200032, China. cjr.guyajia@vip.163.com
- 4Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China.
- KMID: 2413705
- DOI: http://doi.org/10.3348/kjr.2018.19.4.758
Abstract
OBJECTIVE
To investigate the value of diffusion-weighted magnetic resonance imaging (DW-MRI) as a noninvasive tool to assess salivary gland function for follow-up of patients with radiation-induced xerostomia.
MATERIALS AND METHODS
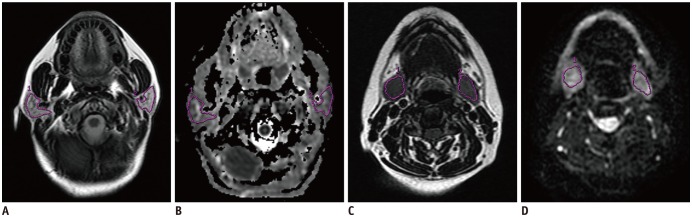
This study included 23 patients with nasopharyngeal carcinoma who had been treated with parotid-sparing radiotherapy (RT). Salivary function was assessed by DW-MRI pre-treatment and one week and one year post-RT, respectively. The maximum apparent diffusion coefficient (ADC) of parotid glands (pADCmax) and the time to peak ADC of parotid glands (pTmax) during stimulation were obtained. Multivariate analysis was used to analyze factors correlated with the severity of radiation-induced xerostomia.
RESULTS
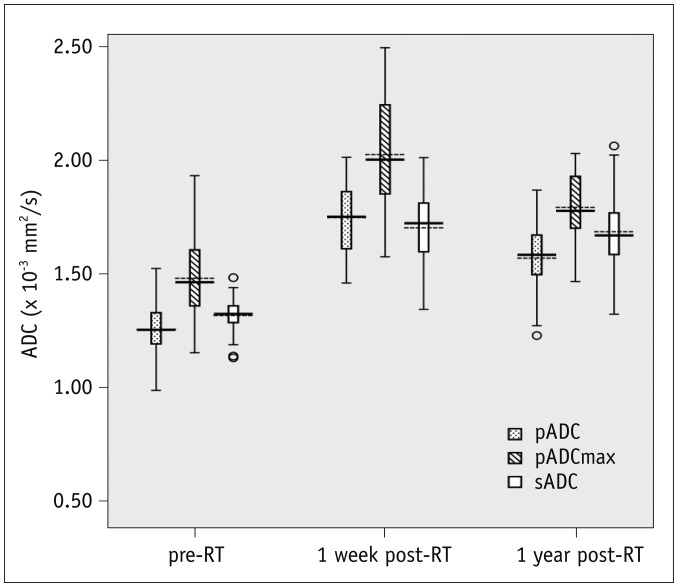
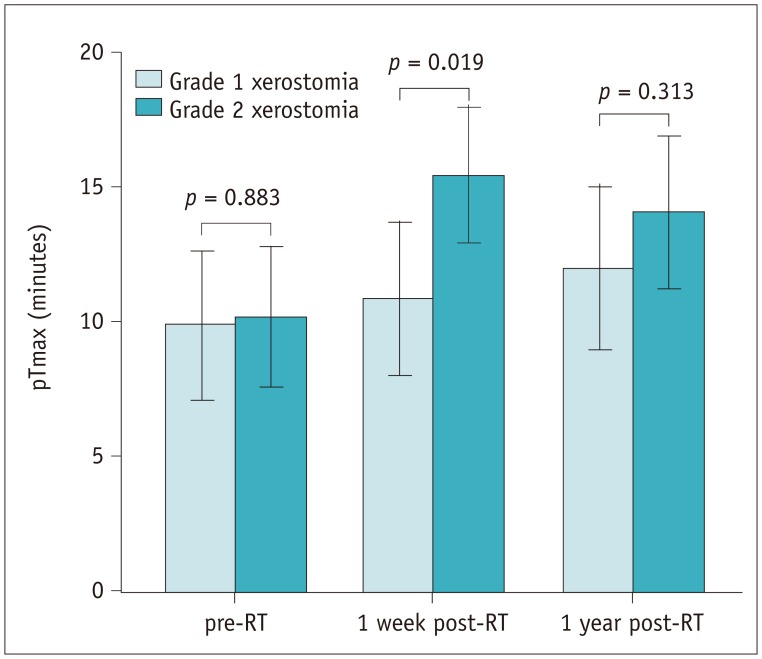
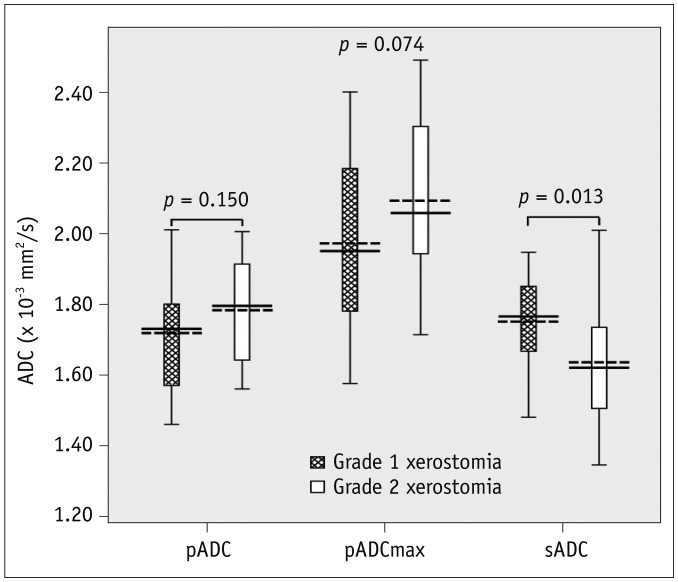
The ADCs of parotid and submandibular glands (1.26 ± 0.10 × 10−3 mm2/s and 1.32 ± 0.07 × 10−3 mm2/s pre-RT, respectively) both showed an increase in all patients at one week post-RT (1.75 ± 0.16 × 10−3 mm2/s, p < 0.001 and 1.70 ± 0.16 × 10−3 mm2/s, p < 0.001, respectively), followed by a decrease in parotid glands at one year post-RT(1.57 ± 0.15 × 10−3 mm2/s, p < 0.001) but not in submandibular glands (1.69 ± 0.18 × 10−3 mm2/s, p = 0.581). An improvement in xerostomia was found in 13 patients at one year post-RT. Multivariate analysis revealed 4 significant predictors for the improvement of xerostomia, including dose to parotid glands (p = 0.009, odds ratio [OR] = 0.639), the ADC of submandibular glands (p = 0.013, OR = 3.295), pADCmax (p = 0.024, OR = 0.474), and pTmax (p = 0.017, OR = 0.729) at one week post-RT.
CONCLUSION
The ADC value is a sensitive indicator for salivary gland dysfunction. DW-MRI is potentially useful for noninvasively predicting the severity of radiation-induced xerostomia.
Keyword
MeSH Terms
Figure
Reference
-
1. Huguenin PU, Taussky D, Moe K, Meister A, Baumert B, Lütolf UM, et al. Quality of life in patients cured from a carcinoma of the head and neck by radiotherapy: the importance of the target volume. Int J Radiat Oncol Biol Phys. 1999; 45:47–52. PMID: 10477005.
Article2. Burlage FR, Coppes RP, Meertens H, Stokman MA, Vissink A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother Oncol. 2001; 61:271–274. PMID: 11730996.
Article3. Saarilahti K, Kouri M, Collan J, Kangasmäki A, Atula T, Joensuu H, et al. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol. 2006; 78:270–275. PMID: 16564589.
Article4. Münter MW, Hoffner S, Hof H, Herfarth KK, Haberkorn U, Rudat V, et al. Changes in salivary gland function after radiotherapy of head and neck tumors measured by quantitative pertechnetate scintigraphy: comparison of intensity-modulated radiotherapy and conventional radiation therapy with and without amifostine. Int J Radiat Oncol Biol Phys. 2007; 67:651–659. PMID: 17175118.
Article5. Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009; 74:1–8. PMID: 19111400.
Article6. Eisbruch A, Rhodus N, Rosenthal D, Murphy B, Rasch C, Sonis S, et al. How should we measure and report radiotherapy-induced xerostomia? Semin Radiat Oncol. 2003; 13:226–234. PMID: 12903012.
Article7. Valdés Olmos RA, Keus RB, Takes RP, van Tinteren H, Baris G, Hilgers FJ, et al. Scintigraphic assessment of salivary function and excretion response in radiation-induced injury of the major salivary glands. Cancer. 1994; 73:2886–2893. PMID: 8199984.
Article8. Habermann CR, Gossrau P, Kooijman H, Graessner J, Cramer MC, Kaul MG, et al. Monitoring of gustatory stimulation of salivary glands by diffusion-weighted MR imaging: comparison of 1.5T and 3T. AJNR Am J Neuroradiol. 2007; 28:1547–1551. PMID: 17846209.
Article9. Ries T, Arndt C, Regier M, Graessner J, Cramer MC, Reitmeier F, et al. Value of apparent diffusion coefficient calculation before and after gustatory stimulation in the diagnosis of acute or chronic parotitis. Eur Radiol. 2008; 18:2251–2257. PMID: 18458907.
Article10. Kato H, Kanematsu M, Toida M, Kawaguchi T, Shibata T, Kajita K, et al. Salivary gland function evaluated by diffusion-weighted MR imaging with gustatory stimulation: preliminary results. J Magn Reson Imaging. 2011; 34:904–909. PMID: 21837780.
Article11. Thoeny HC, De Keyzer F, Boesch C, Hermans R. Diffusion-weighted imaging of the parotid gland: influence of the choice of b-values on the apparent diffusion coefficient value. J Magn Reson Imaging. 2004; 20:786–790. PMID: 15503336.12. Loimu V, Seppälä T, Kapanen M, Tuomikoski L, Nurmi H, Mäkitie A, et al. Diffusion-weighted magnetic resonance imaging for evaluation of salivary gland function in head and neck cancer patients treated with intensity-modulated radiotherapy. Radiother Oncol. 2017; 122:178–184. PMID: 27475276.
Article13. Dirix P, De Keyzer F, Vandecaveye V, Stroobants S, Hermans R, Nuyts S. Diffusion-weighted magnetic resonance imaging to evaluate major salivary gland function before and after radiotherapy. Int J Radiat Oncol Biol Phys. 2008; 71:1365–1371. PMID: 18355977.
Article14. Marzi S, Forina C, Marucci L, Giovinazzo G, Giordano C, Piludu F, et al. Early radiation-induced changes evaluated by intravoxel incoherent motion in the major salivary glands. J Magn Reson Imaging. 2015; 41:974–982. PMID: 24700435.
Article15. Zhang L, Murata Y, Ishida R, Ohashi I, Yoshimura R, Shibuya H. Functional evaluation with intravoxel incoherent motion echo-planar MRI in irradiated salivary glands: a correlative study with salivary gland scintigraphy. J Magn Reson Imaging. 2001; 14:223–229. PMID: 11536398.
Article16. Zhang Y, Ou D, Gu Y, He X, Peng W, Mao J, et al. Diffusion-weighted MR imaging of salivary glands with gustatory stimulation: comparison before and after radiotherapy. Acta Radiol. 2013; 54:928–933. PMID: 23821773.
Article17. Franzén L, Funegård U, Ericson T, Henriksson R. Parotid gland function during and following radiotherapy of malignancies in the head and neck. A consecutive study of salivary flow and patient discomfort. Eur J Cancer. 1992; 28:457–462. PMID: 1591063.18. Stephens LC, Schultheiss TE, Price RE, Ang KK, Peters LJ. Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer. 1991; 67:1539–1543. PMID: 2001542.
Article19. Zeilstra LJ, Vissink A, Konings AW, Coppes RP. Radiation induced cell loss in rat submandibular gland and its relation to gland function. Int J Radiat Biol. 2000; 76:419–429. PMID: 10757322.20. Teshima K, Murakami R, Yoshida R, Nakayama H, Hiraki A, Hirai T, et al. Histopathological changes in parotid and submandibular glands of patients treated with preoperative chemoradiation therapy for oral cancer. J Radiat Res. 2012; 53:492–496. PMID: 22485019.
Article21. Jabbari S, Kim HM, Feng M, Lin A, Tsien C, Elshaikh M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head-and-neck cancer: initial report. Int J Radiat Oncol Biol Phys. 2005; 63:725–731. PMID: 16199308.
Article22. Lin A, Kim HM, Terrell JE, Dawson LA, Ship JA, Eisbruch A. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2003; 57:61–70. PMID: 12909216.
Article23. Coppes RP, Zeilstra LJ, Kampinga HH, Konings AW. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br J Cancer. 2001; 85:1055–1063. PMID: 11592779.
Article24. Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999; 45:577–587. PMID: 10524409.
Article25. Roesink JM, Schipper M, Busschers W, Raaijmakers CP, Terhaard CH. A comparison of mean parotid gland dose with measures of parotid gland function after radiotherapy for head-and-neck cancer: implications for future trials. Int J Radiat Oncol Biol Phys. 2005; 63:1006–1009. PMID: 15964708.
Article26. Chambers MS, Garden AS, Rosenthal D, Ahamad A, Schwartz DL, Blanco AI, et al. Intensity-modulated radiotherapy: is xerostomia still prevalent? Curr Oncol Rep. 2005; 7:131–136. PMID: 15717947.
Article27. Grégoire V, De Neve W, Eisbruch A, Lee N, Van den Weyngaert D, Van Gestel D. Intensity-modulated radiation therapy for head and neck carcinoma. Oncologist. 2007; 12:555–564. PMID: 17522243.
Article28. Wada A, Uchida N, Yokokawa M, Yoshizako T, Kitagaki H. Radiation-induced xerostomia: objective evaluation of salivary gland injury using MR sialography. AJNR Am J Neuroradiol. 2009; 30:53–58. PMID: 18842755.
Article29. Wang J, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001; 220:621–630. PMID: 11526259.
Article30. King AD, Chow KK, Yu KH, Mo FK, Yeung DK, Yuan J, et al. Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology. 2013; 266:531–538. PMID: 23151830.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early Changes in Apparent Diffusion Coefficient for Salivary Glands during Radiotherapy for Nasopharyngeal Carcinoma Associated with Xerostomia
- Diffusion-Weighted Magnetic Resonance Imaging of Spine
- Pituitary Symptomatic Salivary Gland Rest Cyst: Case Report
- Evaluation of Treatment Response Using Diffusion-Weighted MRI in Metastatic Spines
- RE: Diffusion-Weighted Imaging of Prostate Cancer: How Can We Use It Accurately?