Int J Stem Cells.
2018 Jun;11(1):78-86. 10.15283/ijsc17047.
PD-L1 Expression on Circulating CD34+ Hematopoietic Stem Cells Closely Correlated with T-cell Apoptosis in Chronic Hepatitis C Infected Patients
- Affiliations
-
- 1Department of Anatomy and Embryology, Faculty of Medicine, University of Mansoura, Mansoura, Egypt. Hussein.abdullatif@hotmail.com
- 2Department of Anatomy, College of Medicine, University of Bisha, Bisha, Saudi Arabia.
- 3Department of Internal Medicine, Faculty of Medicine, University of Mansoura, Mansoura, Egypt.
- 4Head of Egyptian Liver Research Institute and Hospital (ELRIAH), Mansoura, Egypt.
- KMID: 2413503
- DOI: http://doi.org/10.15283/ijsc17047
Abstract
- BACKGROUND AND OBJECTIVES
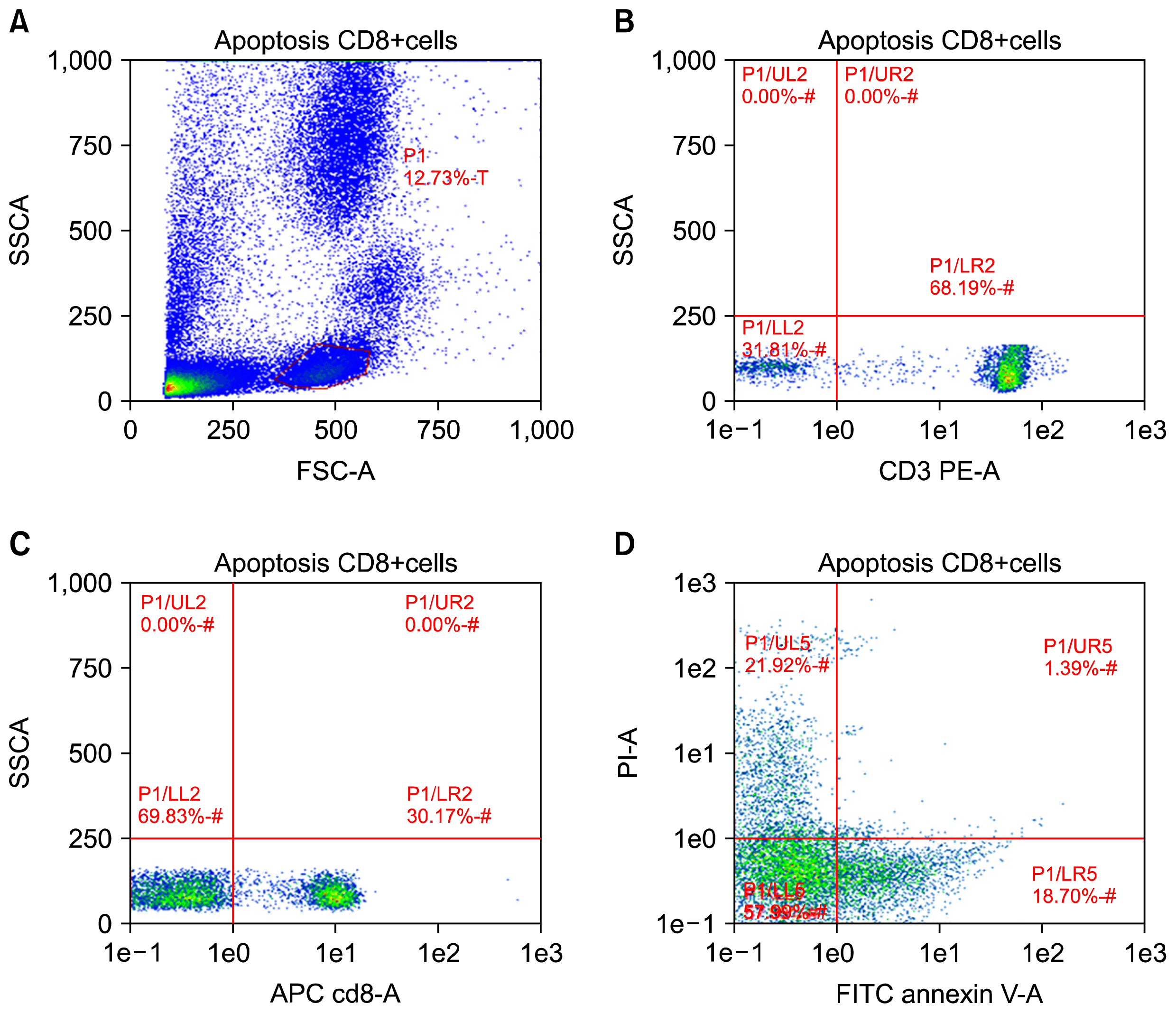
Lack of understanding of the interplay between hematopoietic stem cells (HSCs) and the immune system has severely hampered stem cell research. Programmed death-1 (PD-L1) has been reported on parenchymal cells in patients with chronically inflamed livers and found to play an essential role in T cell homeostasis regulation. However, the bidirectional interaction between HSCs and lymphocytes remains elusive. Here, we aimed to get more insight into circulating CD34+ HSCs PD-L1 expression and T cell apoptosis in chronic HCV infected patients.
METHODS
CD34+ HSCs were isolated and purified by immunomagnetic separation. PD-L1 expression was analyzed by quantitative PCR and flow cytometry. Furthermore, co-culture experiments between CD34+ HSCs and T-lymphocytes were established. T-cell lymphocyte apoptosis in peripheral blood and in cultures was detected.
RESULTS
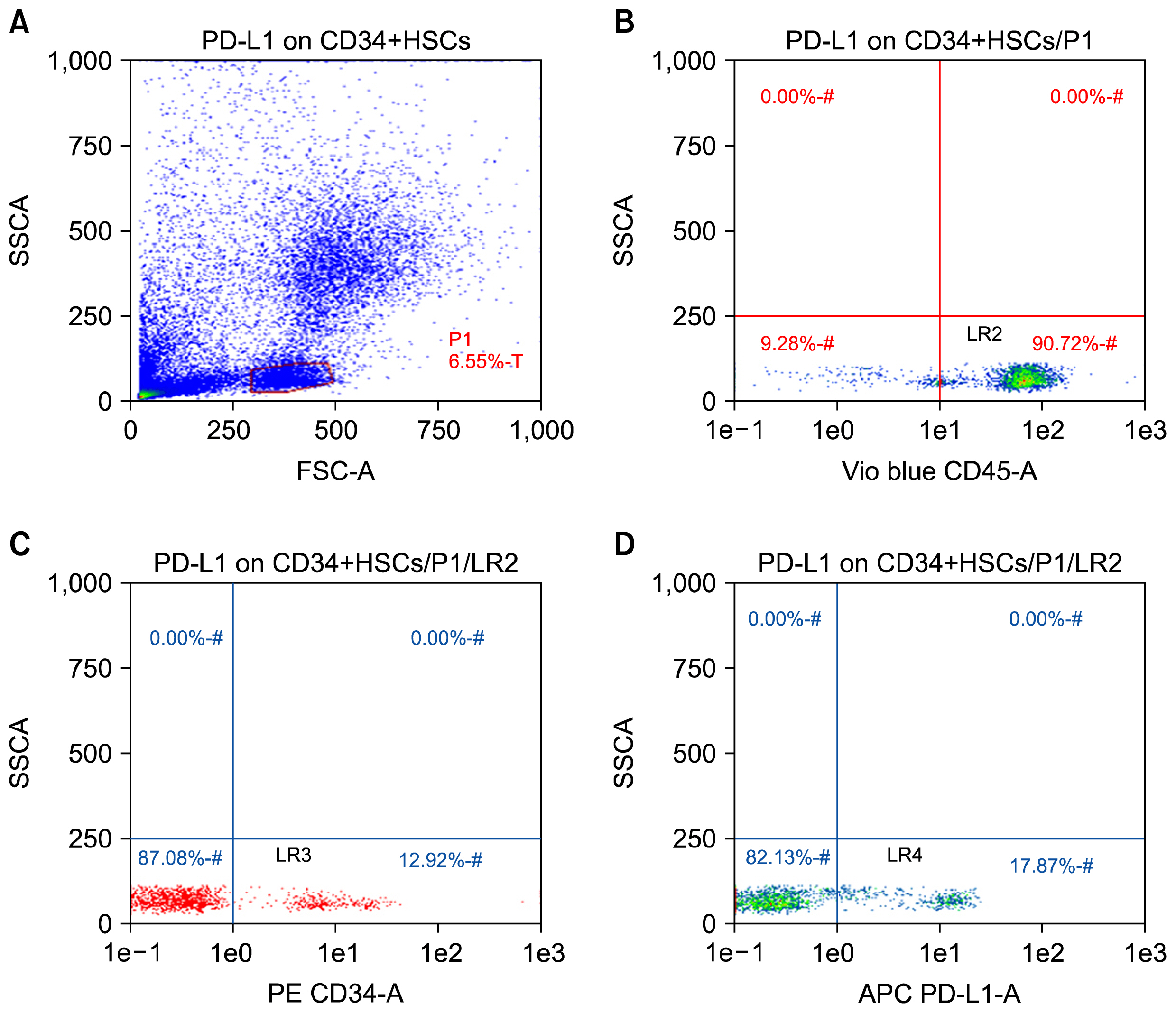
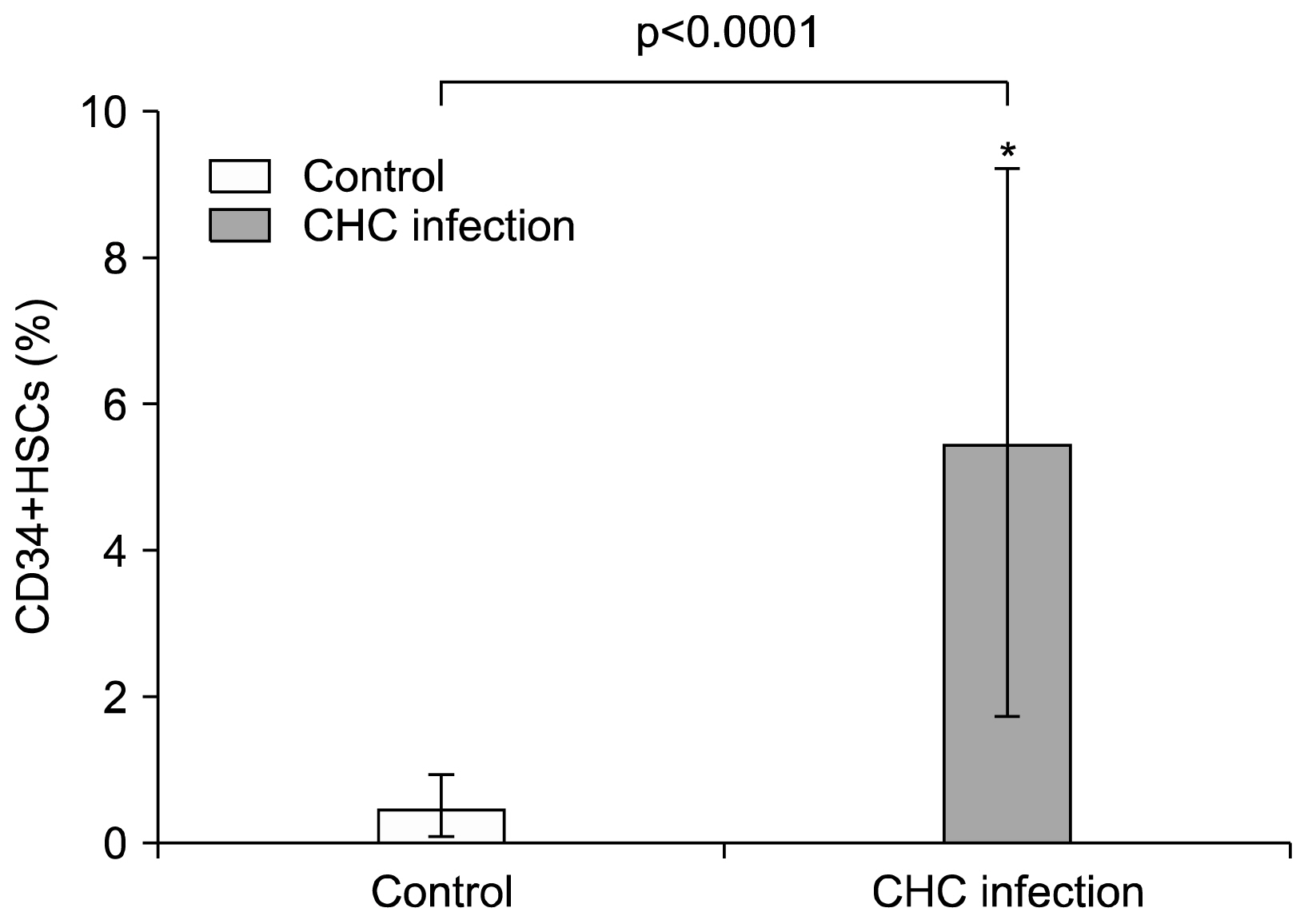
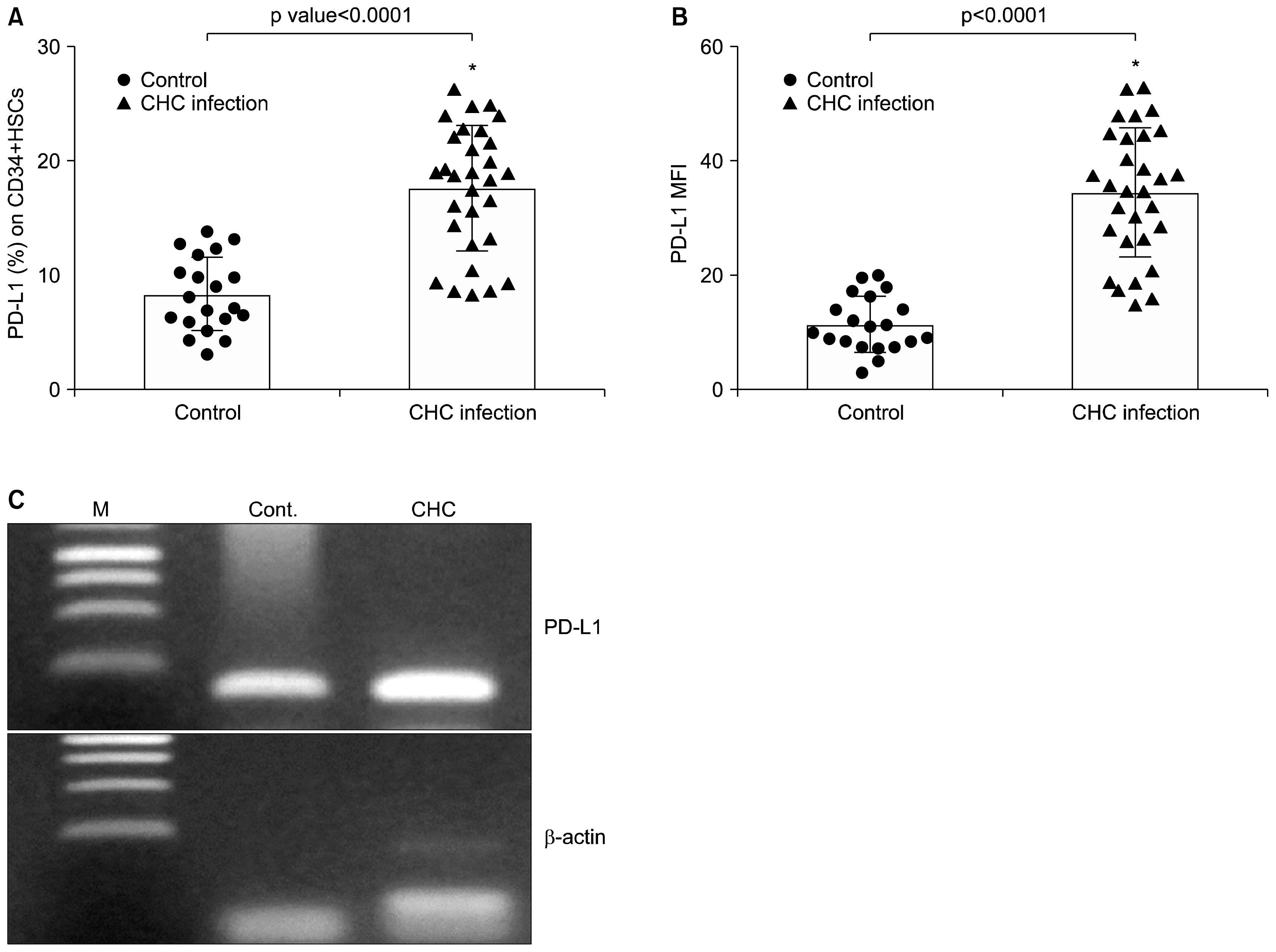
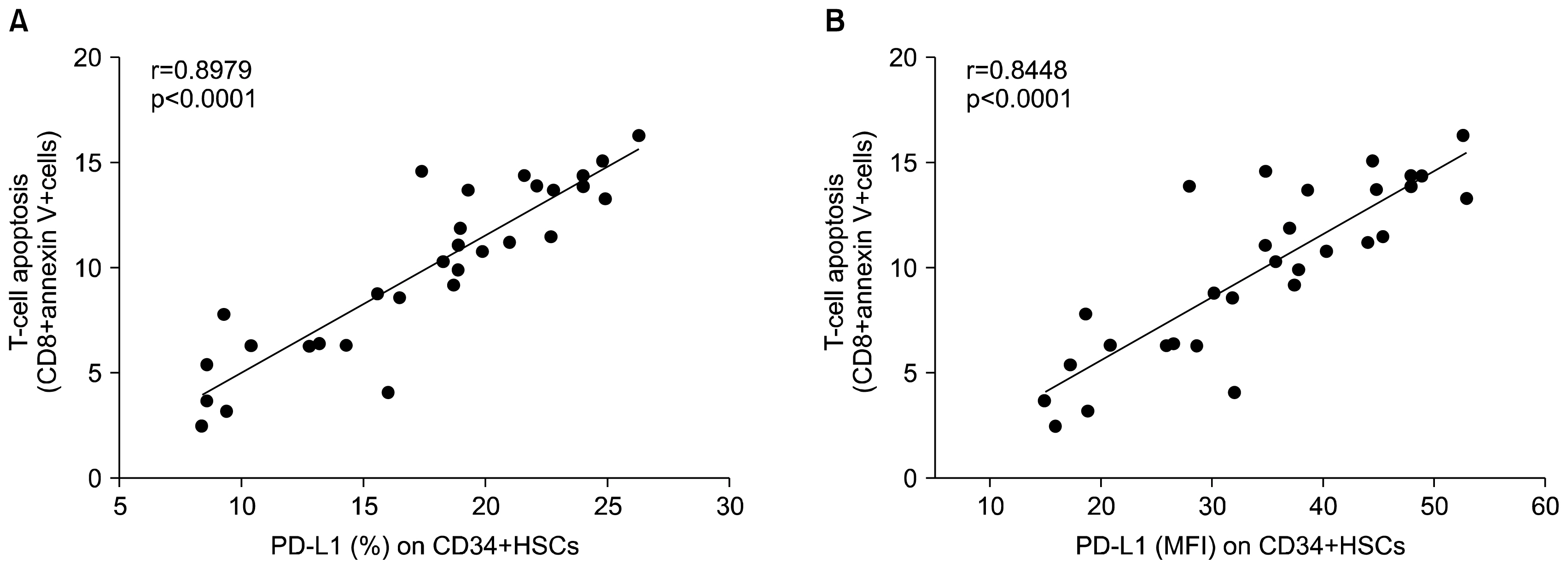
CD34+ HSCs constitutively express low levels of PD-L1. Its expression is up-regulated in chronic HCV infected patients. Moreover, PD-L1 expression on circulating CD34+ HSCs enhanced T cell apoptosis in peripheral blood and co-culture.
CONCLUSION
Our results suggest novel bidirectional interplay between HSCs and lymphocytes mediated by PD-L1 expression on CD34+ HSCs. PD-L1 expression correlated with T-cell lymphocyte apoptosis. This may contribute to immunomodulatory properties of HSCs which improves its use for allogeneic transplantation.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999; 341:556–562. DOI: 10.1056/NEJM199908193410802. PMID: 10451460.
Article2. Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W, Sallam I. The role of parenteral anti-schistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000; 355:887–891. DOI: 10.1016/S0140-6736(99)06527-7.
Article3. Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Goodman ZD. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009; 136:138–148. DOI: 10.1053/j.gastro.2008.09.014.
Article4. McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010; 120:4546–4557. DOI: 10.1172/JCI43127. PMID: 21084749. PMCID: 2994339.
Article5. Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995; 270:1189–1192. DOI: 10.1126/science.270.5239.1189. PMID: 7502042.
Article6. Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999; 5:1365–1369. DOI: 10.1038/70932. PMID: 10581077.
Article7. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000; 192:1027–1034. DOI: 10.1084/jem.192.7.1027. PMID: 11015443. PMCID: 2193311.
Article8. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001; 2:261–268. DOI: 10.1038/85330. PMID: 11224527.
Article9. Oei E, Kalb T, Beuria P, Allez M, Nakazawa A, Azuma M, Timony M, Stuart Z, Chen H, Sperber K. Accessory cell function of airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L318–L331. DOI: 10.1152/ajplung.00174.2003. PMID: 15246982.
Article10. Subudhi SK, Alegre ML, Fu YX. The balance of immune responses: costimulation verse coinhibition. J Mol Med (Berl). 2005; 83:193–202. DOI: 10.1007/s00109-004-0617-1.
Article11. Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004; 20:327–336. DOI: 10.1016/S1074-7613(04)00050-0. PMID: 15030776.
Article12. Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007; 81:9249–9258. DOI: 10.1128/JVI.00409-07. PMID: 17567698. PMCID: 1951397.
Article13. Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007; 81:2545–2553. DOI: 10.1128/JVI.02021-06. PMCID: 1865979.
Article14. Di Campli C, Piscaglia AC, Giuliante F, Rutella S, Bonanno G, Zocco MA, Ardito F, Nuzzo G, Mancuso S, Leone G, Gasbarrini G, Pola P, Gasbarrini A. No evidence of hematopoietic stem cell mobilization in patients submitted to hepatectomy or in patients with acute on chronic liver failure. Transplant Proc. 2005; 37:2563–2566. DOI: 10.1016/j.transproceed.2005.06.072. PMID: 16182744.
Article15. Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003; 198:39–50. DOI: 10.1084/jem.20022235. PMID: 12847136. PMCID: 2196084.
Article16. Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004; 40:1312–1321. DOI: 10.1002/hep.20488. PMID: 15565659.
Article17. Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005; 35:1482–1490. DOI: 10.1002/eji.200425405. PMID: 15827960.
Article18. Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006; 176:2238–2248. DOI: 10.4049/jimmunol.176.4.2238. PMID: 16455980.
Article19. Chironi G, Walch L, Pernollet MG, Gariepy J, Levenson J, Rendu F, Simon A. Decreased number of circulating CD34+KDR+ cells in asymptomatic subjects with preclinical atherosclerosis. Atherosclerosis. 2007; 191:115–120. DOI: 10.1016/j.atherosclerosis.2006.02.041.
Article20. Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003; 33:3117–3126. DOI: 10.1002/eji.200324270. PMID: 14579280.
Article21. Schmidt J, Blum HE, Thimme R. T-cell responses in hepatitis B and C virus infection: similarities and differences. Emerg Microbes Infect. 2013; 2:e15. DOI: 10.1038/emi.2013.14. PMID: 26038456. PMCID: 3630955.
Article22. Zheng J, Umikawa M, Zhang S, Huynh H, Silvany R, Chen BP, Chen L, Zhang CC. Ex vivo expanded hematopoietic stem cells overcome the MHC barrier in allogeneic transplantation. Cell Stem Cell. 2011; 9:119–130. DOI: 10.1016/j.stem.2011.06.003. PMID: 21816363. PMCID: 3151486.
Article23. Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003; 170:1257–1266. DOI: 10.4049/jimmunol.170.3.1257. PMID: 12538684.
Article24. Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, Collins M, Carreno BM. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003; 170:711–718. DOI: 10.4049/jimmunol.170.2.711. PMID: 12517932.
Article25. Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002; 169:3581–3588. DOI: 10.4049/jimmunol.169.7.3581. PMID: 12244148.
Article26. Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, Schölmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006; 45:520–528. DOI: 10.1016/j.jhep.2006.05.007. PMID: 16876901.
Article27. Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002; 169:5538–5545. DOI: 10.4049/jimmunol.169.10.5538. PMID: 12421930.
Article28. Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000; 32:11–16. DOI: 10.1053/jhep.2000.9124. PMID: 10869283.
Article29. Kleeberger W, Rothämel T, Glöckner S, Flemming P, Lehmann U, Kreipe H. High frequency of epithelial chimerism in liver transplants demonstrated by micro-dissection and STR-analysis. Hepatology. 2002; 35:110–116. DOI: 10.1053/jhep.2002.30275. PMID: 11786966.
Article30. Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007; 117:524–529. DOI: 10.1172/JCI31487. PMID: 17332879. PMCID: 1804380.
Article31. Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1: PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. 2007; 178:2714–2720. DOI: 10.4049/jimmunol.178.5.2714. PMID: 17312113.
Article32. Tsuda M, Matsumoto K, Inoue H, Matsumura M, Nakano T, Mori A, Azuma M, Nakanishi Y. Expression of B7-H1 and B7-DC on the airway epithelium is enhanced by double-stranded RNA. Biochem Biophys Res Commun. 2005; 330:263–270. DOI: 10.1016/j.bbrc.2005.02.161. PMID: 15781259.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of circulating PD-1 and PD-L1 as diagnostic biomarkers in dogs with tumors
- The Expression of Fas antigen and Bax and Apoptosis in Ex Vivo Expanded Hematopoietic Progenitor Cells
- Leukotriene B4 pathway regulates the fate of the hematopoietic stem cells
- The Frequency of CD34 and CD34 CD38 Hematopoietic Stem/Progenitor Cells in the Cord Blood and Adult Peripheral Blood
- The Expression of Programmed Death-1 in Circulating CD4+ and CD8+ T Cells during Hepatitis B Virus Infection Progression and Its Correlation with Clinical Baseline Characteristics