Allergy Asthma Immunol Res.
2017 Mar;9(2):169-176. 10.4168/aair.2017.9.2.169.
The Role of IL-17 in a Lipopolysaccharide-Induced Rhinitis Model
- Affiliations
-
- 1Department of Otorhinolaryngology, Dankook University College of Medicine, Cheonan, Korea. jihunmo@gmail.com
- 2Department of Premedical Course, Dankook University College of Medicine, Cheonan, Korea.
- KMID: 2413391
- DOI: http://doi.org/10.4168/aair.2017.9.2.169
Abstract
- PURPOSE
Lipopolysaccharide (LPS) is a cell wall component of Gram-negative bacteria and important for pro-inflammatory mediators. This study aimed to establish a rhinitis model using ovalbumin (OVA) and LPS in order to evaluate the role of interleukin (IL)-17 in the pathogenesis of an LPS-induced non-eosionophilic rhinitis model.
METHODS
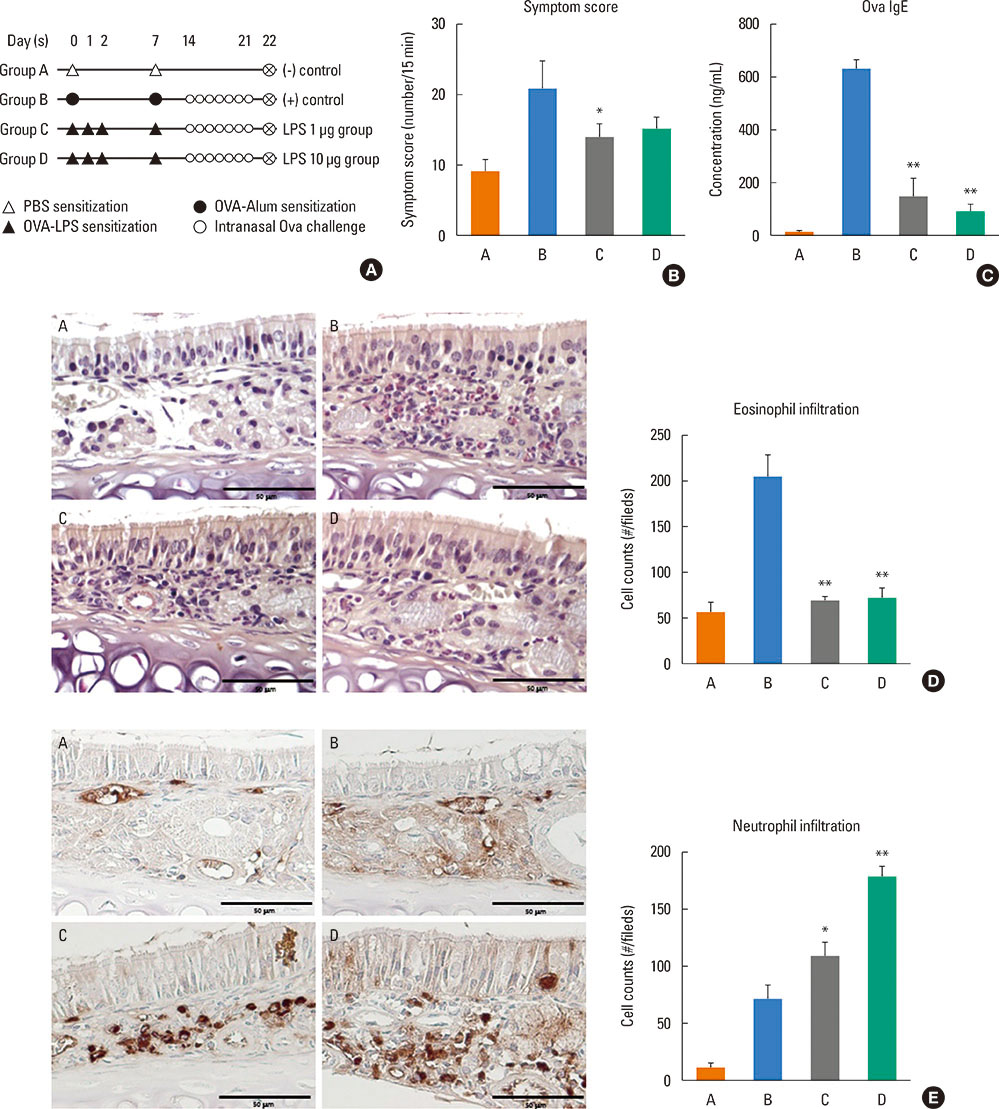
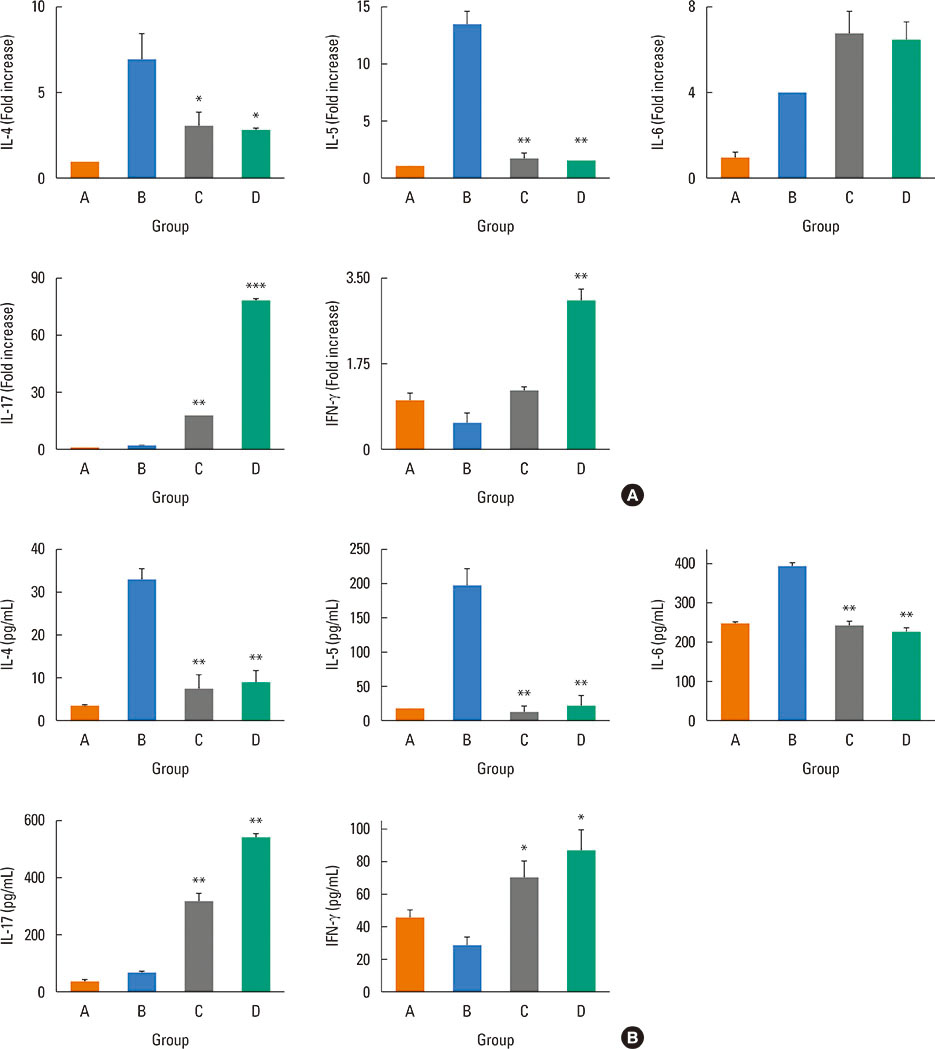
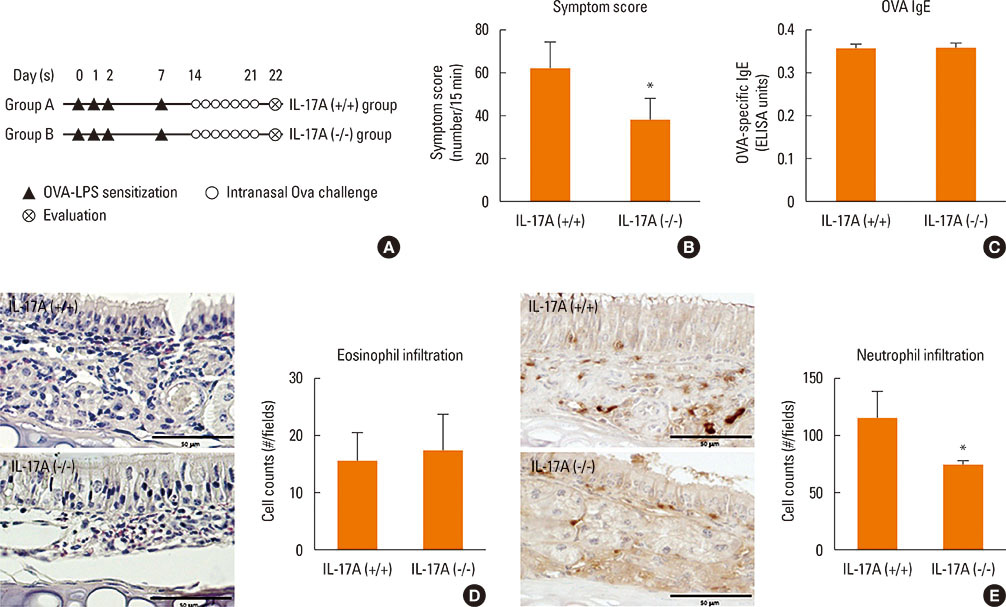
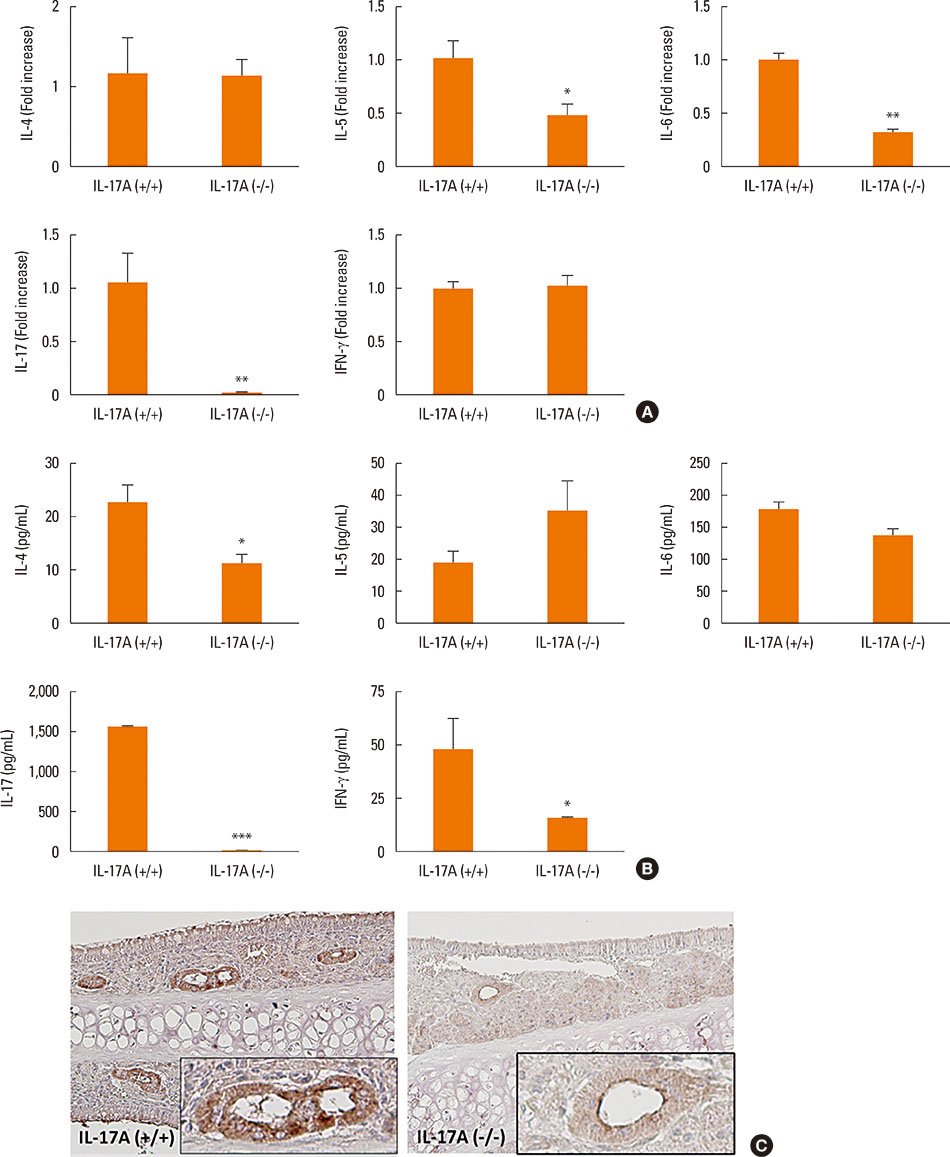
Mice were divided into 4 groups and each group consisted of 10 mice (negative control group, allergic rhinitis model group, 1-µg LPS treatment group, and 10-µg LPS treatment group). BALB/c mice were sensitized with OVA and 1 or 10 µg of LPS, and challenged intranasally with OVA. Multiple parameters of rhinitis were also evaluated to establish the LPS-induced rhinitis model. IL-17 knockout mice were used to check if the LPS-induced rhinitis model were dependent on IL-17. Eosinophil and neutrophil infiltration, and mRNA and protein expression profiles of cytokine in nasal mucosa or spleen cell culture were evaluated using molecular, biochemical, histopathological, and immunohistological methods.
RESULTS
In the LPS-induced rhinitis model, neutrophil infiltration increased in the nasal mucosa, and systemic and nasal IL-17 and interferon-gamma (IFN-γ) levels also increased as compared with the OVA-induced allergic rhinitis model. These findings were LPS-dose-dependent. In IL-17 knockout mice, those phenotypes (neutrophil infiltration, IL-17, and IFN-γ) were reversed, showing IL-17 dependency of LPS-induced rhinitis. The expression of vascular endothelial growth factor (VEGF), an important mediator for inflammation and angiogenesis, decreased in IL-17 knockout mice, showing the relationship between IL-17 and VEGF.
CONCLUSIONS
This study established an LPS-induced rhinitis model dependent on IL-17, characterized by neutrophil infiltration and increased expression of IL-17.
MeSH Terms
-
Animals
Cell Culture Techniques
Cell Wall
Eosinophils
Gram-Negative Bacteria
Inflammation
Interferon-gamma
Interleukin-17*
Interleukins
Mice
Mice, Knockout
Nasal Mucosa
Neutrophil Infiltration
Ovalbumin
Ovum
Phenotype
Rhinitis*
Rhinitis, Allergic
RNA, Messenger
Spleen
Vascular Endothelial Growth Factor A
Interferon-gamma
Interleukin-17
Interleukins
Ovalbumin
RNA, Messenger
Vascular Endothelial Growth Factor A
Figure
Cited by 1 articles
-
Sneezing and Rubbing Counts in Allergic Rhinitis Mouse Models Are a Reliable Indicator of Type 2 Immune Response
Gwanghui Ryu, Jun-Sang Bae, Ji Hye Kim, Eun Hee Kim, Young-Jun Chung, Ji-Hun Mo
Clin Exp Otorhinolaryngol. 2020;13(3):308-311. doi: 10.21053/ceo.2019.02005.
Reference
-
1. Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999; 160:1532–1539.2. Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009; 6:256–259.3. Holgate ST, Holloway J, Wilson S, Howarth PH, Haitchi HM, Babu S, et al. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J Allergy Clin Immunol. 2006; 117:496–506.4. Kämpe M, Stolt I, Lampinen M, Janson C, Stålenheim G, Carlson M. Patients with allergic rhinitis and allergic asthma share the same pattern of eosinophil and neutrophil degranulation after allergen challenge. Clin Mol Allergy. 2011; 9:3.5. Lavinskiene S, Jeroch J, Malakauskas K, Bajoriuniene I, Jackute J, Sakalauskas R. Peripheral blood neutrophil activity during Dermatophagoides pteronyssinus-induced late-phase airway inflammation in patients with allergic rhinitis and asthma. Inflammation. 2012; 35:1600–1609.6. McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008; 181:4089–4097.7. Lindén A, Hoshino H, Laan M. Airway neutrophils and interleukin-17. Eur Respir J. 2000; 15:973–977.8. Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004; 10:1095–1103.9. Mo JH, Chung YJ, Hayashi T, Lee J, Raz E. The role of plasmacytoid and myeloid dendritic cells in induction of asthma in a mouse model and the effect of a TLR9 agonist on dendritic cells. Allergy Asthma Immunol Res. 2011; 3:199–204.10. Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factor-alpha-induced elaboration of proangiogenic factors from fibroblasts. Immunol Lett. 2004; 93:39–43.11. Ryu S, Lee JH, Kim SI. IL-17 increased the production of vascular endothelial growth factor in rheumatoid arthritis synoviocytes. Clin Rheumatol. 2006; 25:16–20.12. Saito H, Matsumoto K, Denburg AE, Crawford L, Ellis R, Inman MD, et al. Pathogenesis of murine experimental allergic rhinitis: a study of local and systemic consequences of IL-5 deficiency. J Immunol. 2002; 168:3017–3023.13. Hellings PW, Hessel EM, Van Den Oord JJ, Kasran A, Van Hecke P, Ceuppens JL. Eosinophilic rhinitis accompanies the development of lower airway inflammation and hyper-reactivity in sensitized mice exposed to aerosolized allergen. Clin Exp Allergy. 2001; 31:782–790.14. Hussain I, Randolph D, Brody SL, Song SK, Hsu A, Kahn AM, et al. Induction, distribution and modulation of upper airway allergic inflammation in mice. Clin Exp Allergy. 2001; 31:1048–1059.15. Mo JH, Park SW, Rhee CS, Takabayashi K, Lee SS, Quan SH, et al. Suppression of allergic response by CpG motif oligodeoxynucleotide-house-dust mite conjugate in animal model of allergic rhinitis. Am J Rhinol. 2006; 20:212–218.16. Tamura S, Kobayashi T, Kikuta K, Nakagawa M, Sakaguchi M, Inouye S. IgE antibody responses against Japanese cedar pollen in the mouse. Microbiol Immunol. 1986; 30:883–891.17. Tsunematsu M, Yamaji T, Kozutsumi D, Murakami R, Kimura S, Kino K. Establishment of an allergic rhinitis model in mice for the evaluation of nasal symptoms. Life Sci. 2007; 80:1388–1394.18. Albano GD, Di Sano C, Bonanno A, Riccobono L, Gagliardo R, Chanez P, et al. Th17 immunity in children with allergic asthma and rhinitis: a pharmacological approach. PLoS One. 2013; 8:e58892.19. Oboki K, Ohno T, Saito H, Nakae S. Th17 and allergy. Allergol Int. 2008; 57:121–134.20. Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Invest. 2004; 114:270–279.21. Kim YS, Hong SW, Choi JP, Shin TS, Moon HG, Choi EJ, et al. Vascular endothelial growth factor is a key mediator in the development of T cell priming and its polarization to type 1 and type 17 T helper cells in the airways. J Immunol. 2009; 183:5113–5120.22. Hata T, Takahashi M, Hida S, Kawaguchi M, Kashima Y, Usui F, et al. Critical role of Th17 cells in inflammation and neovascularization after ischaemia. Cardiovasc Res. 2011; 90:364–372.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mouse Model of IL-17-Dominant Rhinitis Using Polyinosinic-Polycytidylic Acid
- IL-13 and STAT6 signaling involve in low dose lipopolysaccharide induced murine model of asthma
- The Effect of Interleukin-12 on the Immune Response in Allergic Rhinitis Mouse Model
- The Differential Expression of Upper and Lower Airway after Lipopolysaccharide Administration in a Murine Allergy Model
- Allergic Rhinitis Mouse Model