Allergy Asthma Immunol Res.
2017 Mar;9(2):116-125. 10.4168/aair.2017.9.2.116.
Asthma-Related Outcomes in Patients Initiating Extrafine Ciclesonide or Fine-Particle Inhaled Corticosteroids
- Affiliations
-
- 1University of Groningen, Department of Pulmonology, University Medical Center Groningen, Groningen, The Netherlands.
- 2Radboud University Medical Center, Nijmegen, The Netherlands.
- 3Department of Medicine, National Jewish Health, and University of Colorado Denver, Denver, CO, USA.
- 4Emma's Children Hospital AMC, Amsterdam, The Netherlands.
- 5University Paris Descartes (EA2511), Cochin Hospital Group (AP-HP), Paris, France.
- 6Cincinnati Children's Hospital and Medical Center, Cincinnati, OH, USA.
- 7Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
- 8Takeda Pharmaceuticals International GmbH, Zurich, Switzerland.
- 9Takeda Development Centre Europe Ltd, London, UK.
- 10PHARMO Institute for Drug Outcomes Research, The Netherlands.
- 11Research in Real Life, Cambridge, UK. dprice@rirl.org
- 12Academic Primary Care, University of Aberdeen, Aberdeen, UK.
- KMID: 2413385
- DOI: http://doi.org/10.4168/aair.2017.9.2.116
Abstract
- PURPOSE
Extrafine-particle inhaled corticosteroids (ICS) have greater small airway deposition than standard fine-particle ICS. We sought to compare asthma-related outcomes after patients initiated extrafine-particle ciclesonide or fine-particle ICS (fluticasone propionate or non-extrafine beclomethasone).
METHODS
This historical, matched cohort study included patients aged 12-60 years prescribed their first ICS as ciclesonide or fine-particle ICS. The 2 cohorts were matched 1:1 for key demographic and clinical characteristics over the baseline year. Co-primary endpoints were 1-year severe exacerbation rates, risk-domain asthma control, and overall asthma control; secondary endpoints included therapy change.
RESULTS
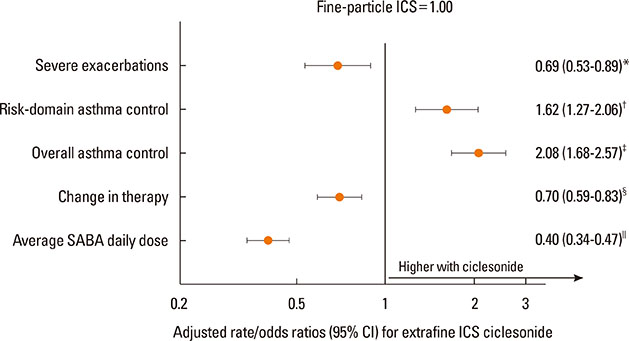
Each cohort included 1,244 patients (median age 45 years; 65% women). Patients in the ciclesonide cohort were comparable to those in the fine-particle ICS cohort apart from higher baseline prevalence of hospitalization, gastroesophageal reflux disease, and rhinitis. Median (interquartile range) prescribed doses of ciclesonide and fine-particle ICS were 160 (160-160) µg/day and 500 (250-500) µg/day, respectively (P<0.001). During the outcome year, patients prescribed ciclesonide experienced lower severe exacerbation rates (adjusted rate ratio [95% CI], 0.69 [0.53-0.89]), and higher odds of risk-domain asthma control (adjusted odds ratio [95% CI], 1.62 [1.27-2.06]) and of overall asthma control (2.08 [1.68-2.57]) than those prescribed fine-particle ICS. The odds of therapy change were 0.70 (0.59-0.83) with ciclesonide.
CONCLUSIONS
In this matched cohort analysis, we observed that initiation of ICS with ciclesonide was associated with better 1-year asthma outcomes and fewer changes to therapy, despite data suggesting more difficult-to-control asthma. The median prescribed dose of ciclesonide was one-third that of fine-particle ICS.
Keyword
MeSH Terms
Figure
Reference
-
1. Global Initiative for Asthma. Global strategy for asthma management and prevention, revised 2014 [Internet]. [place unknown]: Global Initiative for Asthma;2014. cited 2016 Apr 28. Available from: http://www.ginasthma.org.2. British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline (SIGN 141) [Internet]. London: British Thoracic Society;2014. cited 2016 Apr 28. Available from: http://www.sign.ac.uk/pdf/SIGN141.pdf.3. National Heart, Lung, and Blood Institute (US). Expert panel report 3: guidelines for the diagnosis and management of asthma: national asthma education and prevention program [Internet]. Bethesda (MD): National Heart, Lung, and Blood Institute;2007. [cited 2016 Apr 28]. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.4. Usmani OS. Small airways dysfunction in asthma: evaluation and management to improve asthma control. Allergy Asthma Immunol Res. 2014; 6:376–388.5. Lipworth B. Targeting the small airways asthma phenotype: if we can reach it, should we treat it? Ann Allergy Asthma Immunol. 2013; 110:233–239.6. Laube BL, Janssens HM, de Jongh FH, Devadason SG, Dhand R, Diot P, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011; 37:1308–1331.7. Newman S, Salmon A, Nave R, Drollmann A. High lung deposition of 99mTc-labeled ciclesonide administered via HFA-MDI to patients with asthma. Respir Med. 2006; 100:375–384.8. Leach CL, Bethke TD, Boudreau RJ, Hasselquist BE, Drollmann A, Davidson P, et al. Two-dimensional and three-dimensional imaging show ciclesonide has high lung deposition and peripheral distribution: a nonrandomized study in healthy volunteers. J Aerosol Med. 2006; 19:117–126.9. Cohen J, Douma WR, ten Hacken NH, Vonk JM, Oudkerk M, Postma DS. Ciclesonide improves measures of small airway involvement in asthma. Eur Respir J. 2008; 31:1213–1220.10. Hoshino M. Comparison of effectiveness in ciclesonide and fluticasone propionate on small airway function in mild asthma. Allergol Int. 2010; 59:59–66.11. Cohen J, Postma DS, Douma WR, Vonk JM, De Boer AH, ten Hacken NH. Particle size matters: diagnostics and treatment of small airways involvement in asthma. Eur Respir J. 2011; 37:532–540.12. Thompson BR, Douglass JA, Ellis MJ, Kelly VJ, O'Hehir RE, King GG, et al. Peripheral lung function in patients with stable and unstable asthma. J Allergy Clin Immunol. 2013; 131:1322–1328.13. van der Wiel E, ten Hacken NH, Postma DS, van den Berge M. Small-airways dysfunction associates with respiratory symptoms and clinical features of asthma: a systematic review. J Allergy Clin Immunol. 2013; 131:646–657.14. Manoharan A, Anderson WJ, Lipworth J, Ibrahim I, Lipworth BJ. Small airway dysfunction is associated with poorer asthma control. Eur Respir J. 2014; 44:1353–1355.15. Manning P, Gibson PG, Lasserson TJ. Ciclesonide versus other inhaled steroids for chronic asthma in children and adults. Cochrane Database Syst Rev. 2008; CD007031.16. Dahl R, Engelstätter R, Trebas-Pietraś E, Kuna P. A 24-week comparison of low-dose ciclesonide and fluticasone propionate in mild to moderate asthma. Respir Med. 2010; 104:1121–1130.17. Price D, Bjermer L, Popov TA, Chisholm A. Integrating evidence for managing asthma in patients who smoke. Allergy Asthma Immunol Res. 2014; 6:114–120.18. Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ. Influence of particle size and patient dosing technique on lung deposition of HFA-beclomethasone from a metered dose inhaler. J Aerosol Med. 2005; 18:379–385.19. Price D, Bateman ED, Chisholm A, Papadopoulos NG, Bosnic-Anticevich S, Pizzichini E, et al. Complementing the randomized controlled trial evidence base. Evolution not revolution. Ann Am Thorac Soc. 2014; 11:S92–S98.20. Roche N, Reddel HK, Agusti A, Bateman ED, Krishnan JA, Martin RJ, et al. Integrating real-life studies in the global therapeutic research framework. Lancet Respir Med. 2013; 1:e29–e30.21. Price D, Martin RJ, Barnes N, Dorinsky P, Israe lE, Roche N, et al. Prescribing practices and asthma control with hydrofluoroalkanebeclomethasone and fluticasone: a real-world observational study. J Allergy Clin Immunol. 2010; 126:511–518. e1–e10.22. Colice G, Martin RJ, Israel E, Roche N, Barnes N, Burden A, et al. Asthma outcomes and costs of therapy with extrafine beclomethasone and fluticasone. J Allergy Clin Immunol. 2013; 132:45–54.23. PHARMO [Internet]. Utrecht: PHARMO;cited 2016 Apr 28. Available from http://www.pharmo.nl/.24. Herk-Sukel MP, Lemmens VE, Poll-Franse LV, Herings RM, Coebergh JW. Record linkage for pharmacoepidemiological studies in cancer patients. Pharmacoepidemiol Drug Saf. 2012; 21:94–103.25. Herings RM, Pedersen L. Pharmacy-based medical record linkage systems. In : Strom BL, Kimmel SE, Hennessy S, editors. Pharmacoepidemiology. 5th ed. Chichester: Wiley-Blackwell;2012. p. 270–286.26. Roche N, Reddel H, Martin R, Brusselle G, Papi A, Thomas M, et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc. 2014; 11:Suppl 2. S99–S104.27. Respiratory Effectiveness Group (UK) [Internet]. Respiratory Effectiveness Group: Cambridge;cited 2015 Dec 7. Available from: http://www.effectivenessevaluation.org.28. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. The European Union electronic register of post-authorisation studies (EU PAS Register) [Internet]. London: European Network of Centres for Pharmacoepidemiology and Pharmacovigilance;cited 2016 Apr 28. Available from: http://www.encepp.eu/encepp/studiesDatabase.jsp.29. Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009; 180:59–99.30. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010; 25:1–21.31. Sadatsafavi M, Fitz Gerald M, Marra C, Lynd L. Costs and health outcomes associated with primary vs secondary care after an asthma-related hospitalization: a population-based study. Chest. 2013; 144:428–435.32. Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010; 126:466–476.33. Newman SP, Pavia D, Garland N, Clarke SW. Effects of various inhalation modes on the deposition of radioactive pressurized aerosols. Eur J Respir Dis Suppl. 1982; 119:57–65.34. Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005; 172:1497–1504.35. Schatz M, Zeiger RS, Vollmer WM, Mosen D, Mendoza G, Apter AJ, et al. The controller-to-total asthma medication ratio is associated with patient-centered as well as utilization outcomes. Chest. 2006; 130:43–50.36. Roche N, Postma DS, Colice G, Burden A, Guilbert TW, Israel E, et al. Differential effects of inhaled corticosteroids in smokers/exsmokers and nonsmokers with asthma. Am J Respir Crit Care Med. 2015; 191:960–964.37. Schatz M, Zeiger RS, Vollmer WM, Mosen D, Apter AJ, Stibolt TB, et al. Validation of a beta-agonist long-term asthma control scale derived from computerized pharmacy data. J Allergy Clin Immunol. 2006; 117:995–1000.38. Price D, Chrystyn H, Kaplan A, Haughney J, Román-Rodríguez M, Burden A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012; 4:184–191.39. Black PN, Lawrence BJ, Goh KH, Barry MS. Differences in the potencies of inhaled steroids are not reflected in the doses prescribed in primary care in New Zealand. Eur J Clin Pharmacol. 2000; 56:431–435.40. Schirm E, de Vries TW, Tobi H, van den Berg PB, de Jong-van den Berg LT. Prescribed doses of inhaled steroids in Dutch children: too little or too much, for too short a time. Br J Clin Pharmacol. 2006; 62:383–390.41. van der Molen T, Foster JM, Caeser M, Müller T, Postma DS. Difference between patient-reported side effects of ciclesonide versus fluticasone propionate. Respir Med. 2010; 104:1825–1833.42. McKeever T, Harrison TW, Hubbard R, Shaw D. Inhaled corticosteroids and the risk of pneumonia in people with asthma: a casecontrol study. Chest. 2013; 144:1788–1794.43. Nave R, Watz H, Hoffmann H, Boss H, Magnussen H. Deposition and metabolism of inhaled ciclesonide in the human lung. Eur Respir J. 2010; 36:1113–1119.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhaled Corticosteroids Is Not Associated with the Risk of Pneumonia in Asthma
- Treatment of mild asthma: Is it necessary to keep regular inhaled corticosteroids?
- An experimental asthma exacerbation following discontinuation of inhaled corticosteroids treatment in patients with controlled asthma
- Economic Evaluations of Alternative Inhaled Steroid Medications in Patients with Asthma
- Add-on Therapy for Symptomatic Asthma despite Long-Acting Beta-Agonists/Inhaled Corticosteroid