Lobeglitazone, a Novel Peroxisome Proliferator-Activated Receptor γ Agonist, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
- Affiliations
-
- 1Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea. kpark@knu.ac.kr mine9240@naver.com
- 2Leading-Edge Research Center for Drug Discovery and Development for Diabetes and Metabolic Disease, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea.
- 3Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 4Department of Surgery, Keimyung University School of Medicine, Daegu, Korea.
- 5Department of Biochemistry and Cell Biology, Cell and Matrix Research Institute, Kyungpook National University School of Medicine, Daegu, Korea.
- 6New Drug Development Center, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea.
- KMID: 2413295
- DOI: http://doi.org/10.3803/EnM.2017.32.1.115
Abstract
- BACKGROUND
Renal tubulointerstitial fibrosis is a common feature of the final stage of nearly all cause types of chronic kidney disease. Although classic peroxisome proliferator-activated receptor γ (PPARγ) agonists have a protective effect on diabetic nephropathy, much less is known about their direct effects in renal fibrosis. This study aimed to investigate possible beneficial effects of lobeglitazone, a novel PPARγ agonist, on renal fibrosis in mice.
METHODS
We examined the effects of lobeglitazone on renal tubulointerstitial fibrosis in unilateral ureteral obstruction (UUO) induced renal fibrosis mice. We further defined the role of lobeglitazone on transforming growth factor (TGF)-signaling pathways in renal tubulointerstitial fibrosis through in vivo and in vitro study.
RESULTS
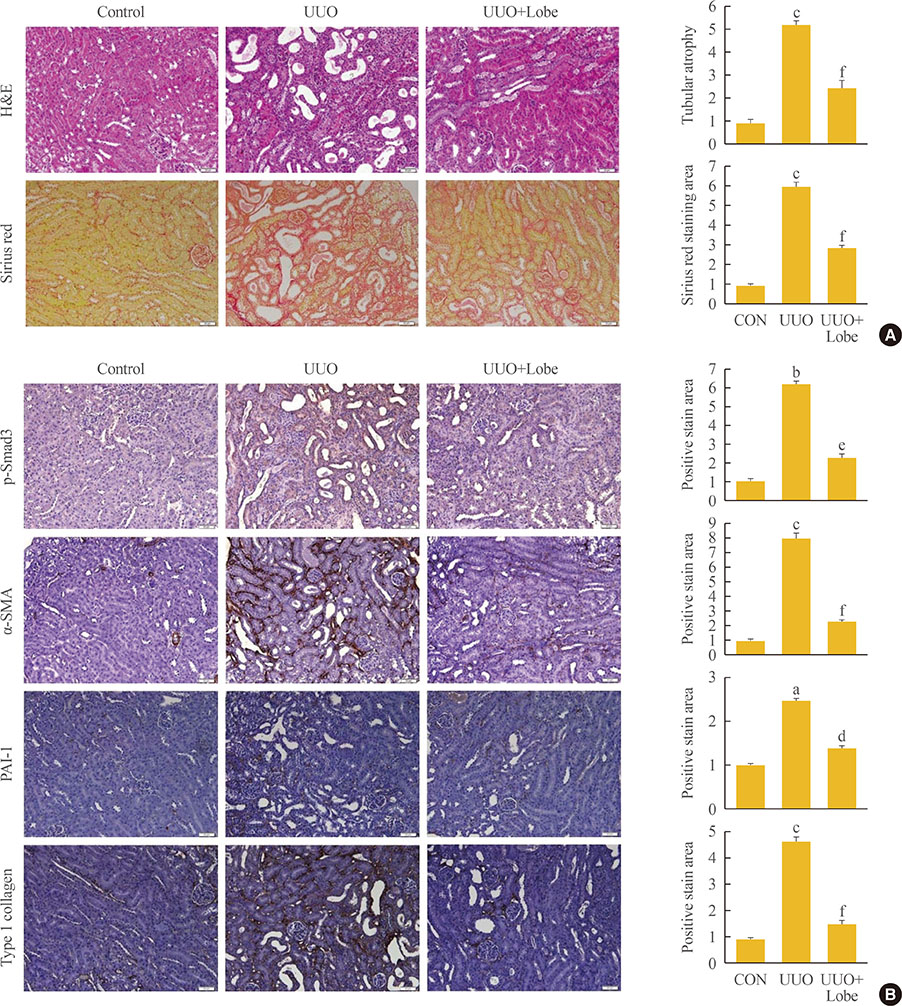
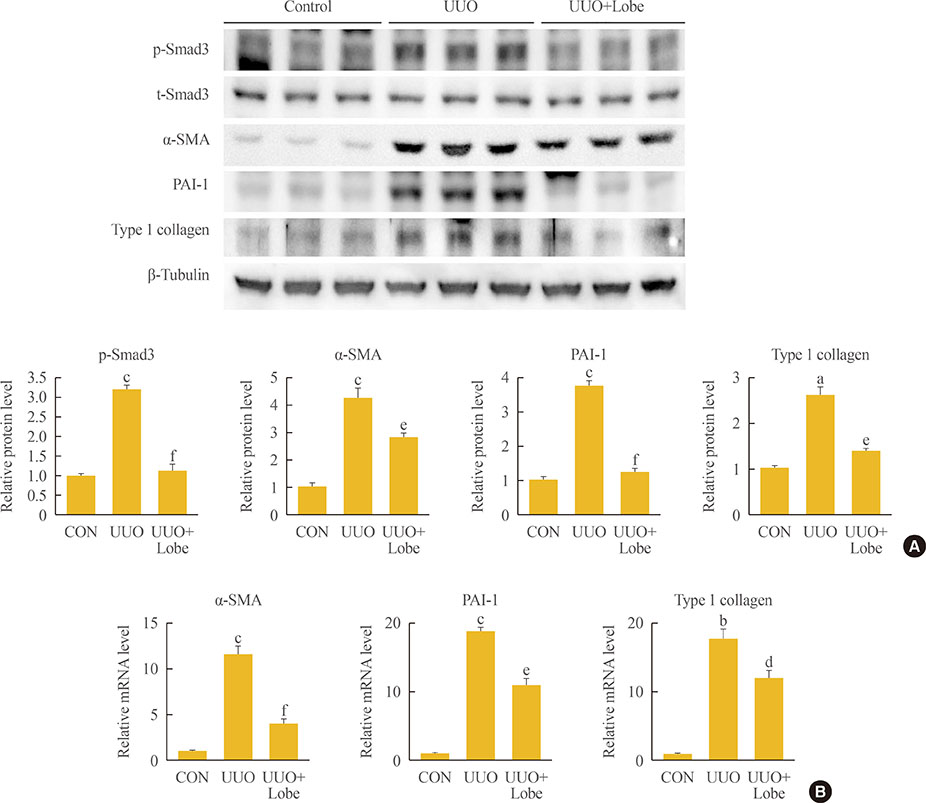
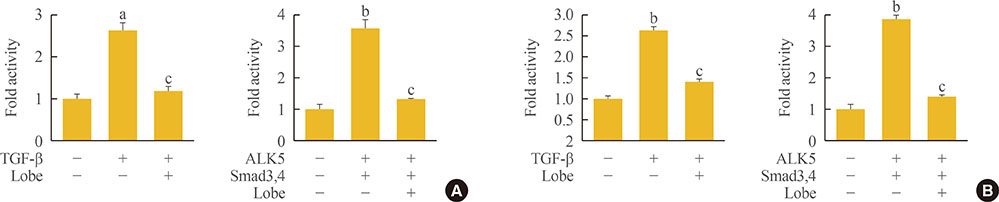
Through hematoxylin/eosin and sirius red staining, we observed that lobeglitazone effectively attenuates UUO-induced renal atrophy and fibrosis. Immunohistochemical analysis in conjunction with quantitative reverse transcription polymerase chain reaction and Western blot analysis revealed that lobeglitazone treatment inhibited UUO-induced upregulation of renal Smad-3 phosphorylation, α-smooth muscle actin, plasminogen activator inhibitor 1, and type 1 collagen. In vitro experiments with rat mesangial cells and NRK-49F renal fibroblast cells suggested that the effects of lobeglitazone on UUO-induced renal fibrosis are mediated by inhibition of the TGF-β/Smad signaling pathway.
CONCLUSION
The present study demonstrates that lobeglitazone has a protective effect on UUO-induced renal fibrosis, suggesting that its clinical applications could extend to the treatment of non-diabetic origin renal disease.
Keyword
MeSH Terms
-
Actins
Animals
Atrophy
Blotting, Western
Collagen Type I
Diabetic Nephropathies
Fibroblasts
Fibrosis*
In Vitro Techniques
Mesangial Cells
Mice*
Peroxisomes*
Phosphorylation
Plasminogen Activator Inhibitor 1
Polymerase Chain Reaction
Rats
Renal Insufficiency, Chronic
Reverse Transcription
Transforming Growth Factor beta
Transforming Growth Factors
Up-Regulation
Ureter*
Ureteral Obstruction*
Actins
Collagen Type I
Plasminogen Activator Inhibitor 1
Transforming Growth Factor beta
Transforming Growth Factors
Figure
Cited by 3 articles
-
Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
Diabetes Metab J. 2021;45(3):326-336. doi: 10.4093/dmj.2020.0272.Effects of Lobeglitazone, a Novel Thiazolidinedione, on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus over 52 Weeks
Soo Lim, Kyoung Min Kim, Sin Gon Kim, Doo Man Kim, Jeong-Taek Woo, Choon Hee Chung, Kyung Soo Ko, Jeong Hyun Park, Yongsoo Park, Sang Jin Kim, Hak Chul Jang, Dong Seop Choi
Diabetes Metab J. 2017;41(5):377-385. doi: 10.4093/dmj.2017.41.5.377.Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing p38 MAPK Signaling Pathway
Jun-Qing Jin, Jeong-Sun Han, Jeonghoon Ha, Han-Sang Baek, Dong-Jun Lim
Endocrinol Metab. 2021;36(5):1095-1110. doi: 10.3803/EnM.2021.1155.
Reference
-
1. Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005; 365:331–340.2. Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010; 21:1819–1834.3. Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006; 17:17–25.4. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016; 12:325–338.5. Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006; 17:2999–3012.6. Jung GS, Kim MK, Jung YA, Kim HS, Park IS, Min BH, et al. Clusterin attenuates the development of renal fibrosis. J Am Soc Nephrol. 2012; 23:73–85.7. Won JC, Park CY, Oh SW, Lee ES, Youn BS, Kim MS. Plasma clusterin (ApoJ) levels are associated with adiposity and systemic inflammation. PLoS One. 2014; 9:e103351.8. Derosa G, Maffioli P. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) agonists on glycemic control, lipid profile and cardiovascular risk. Curr Mol Pharmacol. 2012; 5:272–281.9. Cunard R, Ricote M, DiCampli D, Archer DC, Kahn DA, Glass CK, et al. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. J Immunol. 2002; 168:2795–2802.10. Guan Y, Zhang Y, Breyer MD. The role of PPARs in the transcriptional control of cellular processes. Drug News Perspect. 2002; 15:147–154.11. Smith SA, Lister CA, Toseland CD, Buckingham RE. Rosiglitazone prevents the onset of hyperglycaemia and proteinuria in the Zucker diabetic fatty rat. Diabetes Obes Metab. 2000; 2:363–372.12. Ma LJ, Marcantoni C, Linton MF, Fazio S, Fogo AB. Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int. 2001; 59:1899–1910.13. Routh RE, Johnson JH, McCarthy KJ. Troglitazone suppresses the secretion of type I collagen by mesangial cells in vitro. Kidney Int. 2002; 61:1365–1376.14. Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M. Peroxisome proliferator-activated receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin expression in glomerular mesangial cells. Diabetes. 2004; 53:200–208.15. Kim JW, Kim JR, Yi S, Shin KH, Shin HS, Yoon SH, et al. Tolerability and pharmacokinetics of lobeglitazone (CKD-501), a peroxisome proliferator-activated receptor-gamma agonist: a single- and multiple-dose, double-blind, randomized control study in healthy male Korean subjects. Clin Ther. 2011; 33:1819–1830.16. Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, Ko KS, et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS One. 2014; 9:e92843.17. Jin SM, Park CY, Cho YM, Ku BJ, Ahn CW, Cha BS, et al. Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension. Diabetes Obes Metab. 2015; 17:599–602.18. Lim S, Lee KS, Lee JE, Park HS, Kim KM, Moon JH, et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015; 243:107–119.19. Jung GS, Jeon JH, Choe MS, Kim SW, Lee IK, Kim MK, et al. Renoprotective effect of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in streptozotocin-induced type 1 diabetic mice. Diabetes Metab J. 2016; 40:211–221.20. Jung GS, Kim MK, Choe MS, Lee KM, Kim HS, Park YJ, et al. The orphan nuclear receptor SHP attenuates renal fibrosis. J Am Soc Nephrol. 2009; 20:2162–2170.21. Kim H, Haluzik M, Gavrilova O, Yakar S, Portas J, Sun H, et al. Thiazolidinediones improve insulin sensitivity in adipose tissue and reduce the hyperlipidaemia without affecting the hyperglycaemia in a transgenic model of type 2 diabetes. Diabetologia. 2004; 47:2215–2225.22. Pistrosch F, Passauer J, Herbrig K, Schwanebeck U, Gross P, Bornstein SR. Effect of thiazolidinedione treatment on proteinuria and renal hemodynamic in type 2 diabetic patients with overt nephropathy. Horm Metab Res. 2012; 44:914–918.23. Benigni A, Zoja C, Tomasoni S, Campana M, Corna D, Zanchi C, et al. Transcriptional regulation of nephrin gene by peroxisome proliferator-activated receptor-gamma agonist: molecular mechanism of the antiproteinuric effect of pioglitazone. J Am Soc Nephrol. 2006; 17:1624–1632.24. Jesse CR, Bortolatto CF, Wilhelm EA, Roman SS, Prigol M, Nogueira CW. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone protects against cisplatin-induced renal damage in mice. J Appl Toxicol. 2014; 34:25–32.25. Doi S, Masaki T, Arakawa T, Takahashi S, Kawai T, Nakashima A, et al. Protective effects of peroxisome proliferator-activated receptor gamma ligand on apoptosis and hepatocyte growth factor induction in renal ischemia-reperfusion injury. Transplantation. 2007; 84:207–213.26. Lin Q, Gu Y, Ma J, Lin SY. Protection of rosiglitazone against renal interstitial lesion and its mechanism. Zhonghua Yi Xue Za Zhi. 2005; 85:1618–1624.27. Han JY, Kim YJ, Kim L, Choi SJ, Park IS, Kim JM, et al. PPARgamma agonist and angiotensin II receptor antagonist ameliorate renal tubulointerstitial fibrosis. J Korean Med Sci. 2010; 25:35–41.28. Higashi K, Oda T, Kushiyama T, Hyodo T, Yamada M, Suzuki S, et al. Additive antifibrotic effects of pioglitazone and candesartan on experimental renal fibrosis in mice. Nephrology (Carlton). 2010; 15:327–335.29. Kawai T, Masaki T, Doi S, Arakawa T, Yokoyama Y, Doi T, et al. PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Lab Invest. 2009; 89:47–58.30. Lan HY, Chung AC. TGF-beta/Smad signaling in kidney disease. Semin Nephrol. 2012; 32:236–243.31. Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, et al. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol. 2010; 21:1477–1487.32. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004; 18:816–827.33. Ohtomo S, Izuhara Y, Takizawa S, Yamada N, Kakuta T, van Ypersele de Strihou C, et al. Thiazolidinediones provide better renoprotection than insulin in an obese, hypertensive type II diabetic rat model. Kidney Int. 2007; 72:1512–1519.34. Bae EH, Kim IJ, Ma SK, Kim SW. Rosiglitazone prevents the progression of renal injury in DOCA-salt hypertensive rats. Hypertens Res. 2010; 33:255–262.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PPARgamma Agonist and Angiotensin II Receptor Antagonist Ameliorate Renal Tubulointerstitial Fibrosis
- Peroxisome Proliferator-activated Receptors (PPARs) in Diabetic Nephropathy
- Peroxisome Proliferator-Activated Receptor Gamma Agonist Attenuates Liver Fibrosis by Several Fibrogenic Pathways in an Animal Model of Cholestatic Fibrosis
- 17beta-estradiol Attenuates Renal Fibrosis in Mice with Obstructive Uropathy
- Refocusing Peroxisome Proliferator Activated Receptor-alpha: A New Insight for Therapeutic Roles in Diabetes