Clin Exp Otorhinolaryngol.
2018 Jun;11(2):124-132. 10.21053/ceo.2017.00493.
Clusterin Induces MUC5AC Expression via Activation of NF-κB in Human Airway Epithelial Cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Yeungnam University College of Medicine, Daegu, Korea. ydkim@med.yu.ac.kr
- 2Regional Center for Respiratory Diseases, Yeungnam University Medical Center, Daegu, Korea.
- KMID: 2412670
- DOI: http://doi.org/10.21053/ceo.2017.00493
Abstract
OBJECTIVES
Clusterin (CLU) is known as apolipoprotein J, and has three isoforms with different biological functions. CLU is associated with various diseases such as Alzheimer disease, atherosclerosis, and some malignancies. Recent studies report an association of CLU with inflammation and immune response in inflammatory airway diseases. However, the effect of CLU on mucin secretion of airway epithelial cells has not yet been understood. Therefore, the effect and brief signaling pathway of CLU on MUC5AC (as a major secreted mucin) expression were investigated in human airway epithelial cells.
METHODS
In the tissues of nasal polyp and normal inferior turbinate, the presence of MUC5AC and CLU was investigated using immunohistochemical stain and Western blot analysis. In mucin-producing human NCI-H292 airway epithelial cells and primary cultures of normal nasal epithelial cells, the effect and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway of CLU on MUC5AC expression were investigated using immunohistochemical stain, reverse transcription-polymerase chain reaction, real-time polymerase chain reaction, enzyme immunoassay, and Western blot analysis.
RESULTS
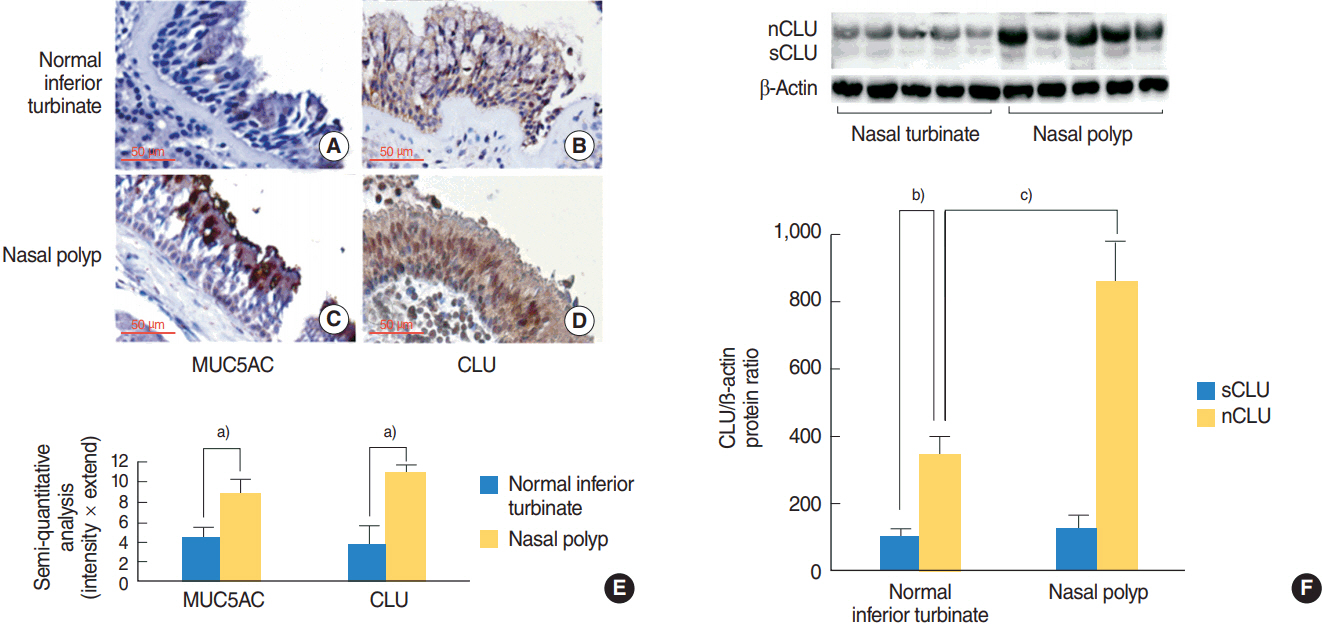
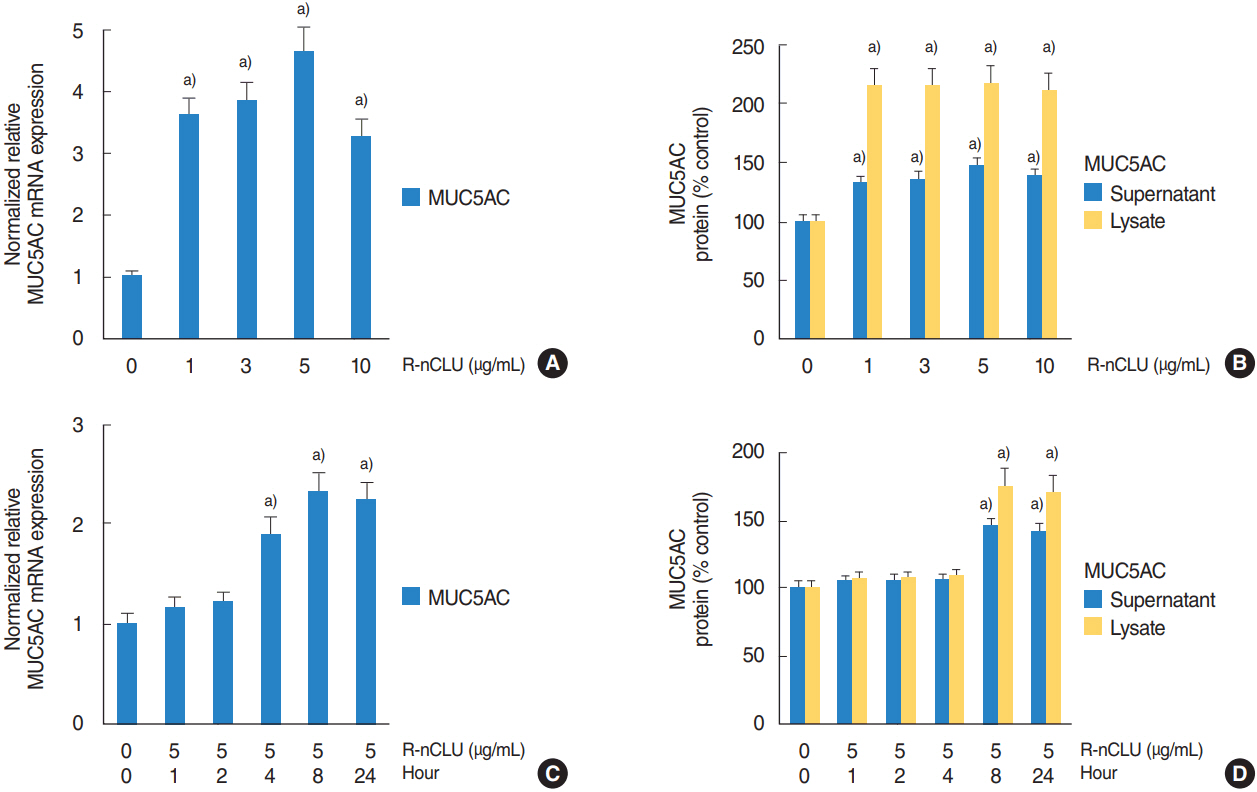
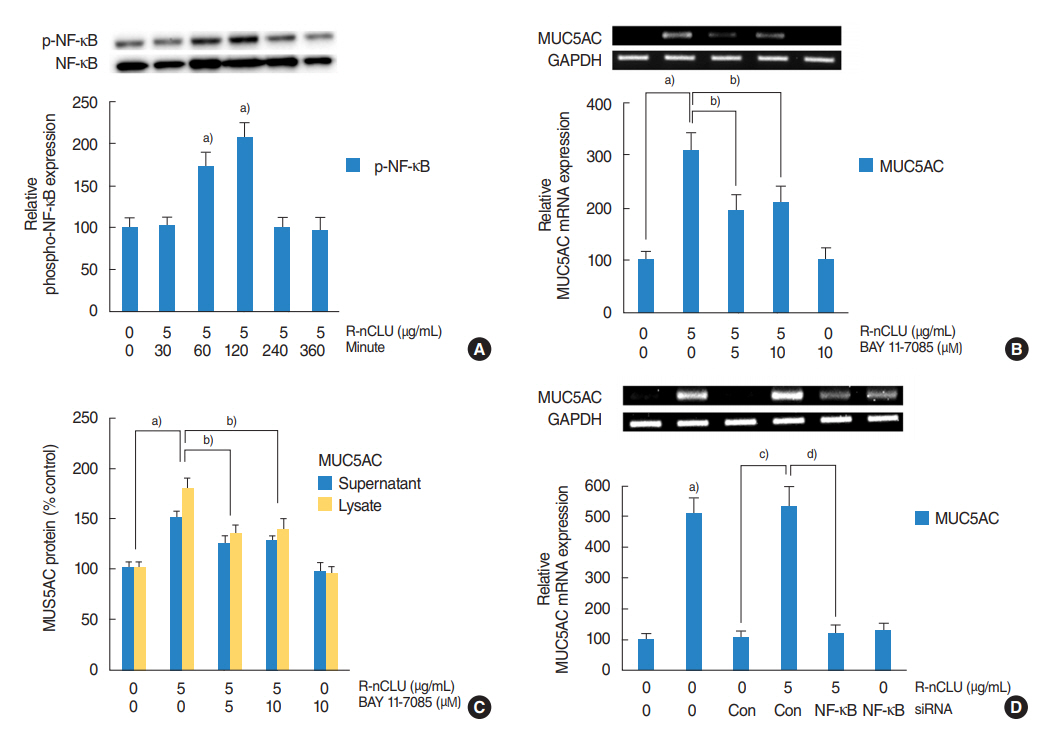
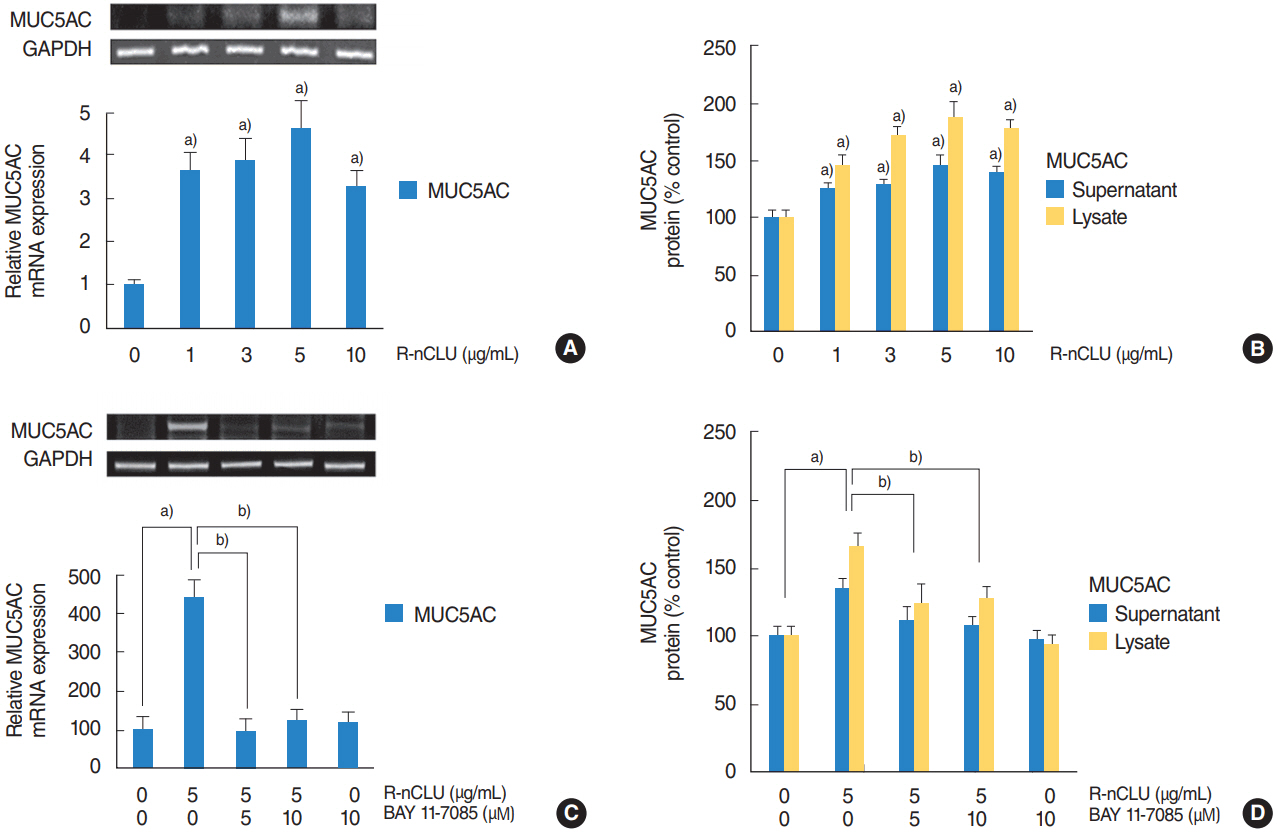
In the nasal polyps, MUC5AC and CLU were abundantly present in the epithelium on immunohistochemical stain, and nuclear CLU (nCLU) was strongly detected on Western blot analysis. In human NCI-H292 airway epithelial cells or the primary cultures of normal nasal epithelial cells, recombinant nCLU increased MUC5AC expression, and significantly activated phosphorylation of NF-κB. And BAY 11-7085 (a specific NF-κB inhibitor) and knockdown of NF-κB by NF-κB siRNA (small interfering RNA) significantly attenuated recombinant nCLU-induced MUC5AC expression.
CONCLUSION
These results suggest that nCLU induces MUC5AC expression via the activation of NF-κB signaling pathway in human airway epithelial cells.
Keyword
MeSH Terms
-
Alzheimer Disease
Atherosclerosis
B-Lymphocytes
Bays
Blotting, Western
Clusterin*
Epithelial Cells*
Epithelium
Humans*
Immunoenzyme Techniques
Inflammation
Mucins
Nasal Polyps
NF-kappa B
Phosphorylation
Protein Isoforms
Real-Time Polymerase Chain Reaction
RNA, Small Interfering
Turbinates
Clusterin
Mucins
NF-kappa B
Protein Isoforms
RNA, Small Interfering
Figure
Reference
-
1. Pawankar R, Nonaka M. Inflammatory mechanisms and remodeling in chronic rhinosinusitis and nasal polyps. Curr Allergy Asthma Rep. 2007; Jun. 7(3):202–8.
Article2. Kim HK, Kook JH, Kang KR, Oh DJ, Kim TH, Lee SH. Increased expression of hCLCA1 in chronic rhinosinusitis and its contribution to produce MUC5AC. Laryngoscope. 2016; Nov. 126(11):E347–55.
Article3. Ha EV, Rogers DF. Novel therapies to inhibit mucus synthesis and secretion in airway hypersecretory diseases. Pharmacology. 2016; Jan. 97(1-2):84–100.
Article4. Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009; Feb. 135(2):505–12.
Article5. Jeong S, Ledee DR, Gordon GM, Itakura T, Patel N, Martin A, et al. Interaction of clusterin and matrix metalloproteinase-9 and its implication for epithelial homeostasis and inflammation. Am J Pathol. 2012; May. 180(5):2028–39.
Article6. Park S, Mathis KW, Lee IK. The physiological roles of apolipoprotein J/clusterin in metabolic and cardiovascular diseases. Rev Endocr Metab Disord. 2014; Mar. 15(1):45–53.
Article7. Sol IS, Kim YH, Park YA, Lee KE, Hong JY, Kim MN, et al. Relationship between sputum clusterin levels and childhood asthma. Clin Exp Allergy. 2016; May. 46(5):688–95.
Article8. Kwon HS, Kim TB, Lee YS, Jeong SH, Bae YJ, Moon KA, et al. Clusterin expression level correlates with increased oxidative stress in asthmatics. Ann Allergy Asthma Immunol. 2014; Mar. 112(3):217–21.
Article9. Hong GH, Kwon HS, Moon KA, Park SY, Park S, Lee KY, et al. Clusterin modulates allergic airway inflammation by attenuating CCL20-mediated dendritic cell recruitment. J Immunol. 2016; Mar. 196(5):2021–30.
Article10. Carnevali S, Luppi F, D’Arca D, Caporali A, Ruggieri MP, Vettori MV, et al. Clusterin decreases oxidative stress in lung fibroblasts exposed to cigarette smoke. Am J Respir Crit Care Med. 2006; Aug. 174(4):393–9.
Article11. Reddy KB, Karode MC, Harmony AK, Howe PH. Interaction of transforming growth factor beta receptors with apolipoprotein J/clusterin. Biochemistry. 1996; Jan. 35(1):309–14.12. Santilli G, Aronow BJ, Sala A. Essential requirement of apolipoprotein J (clusterin) signaling for IkappaB expression and regulation of NF-kappaB activity. J Biol Chem. 2003; Oct. 278(40):38214–9.13. Mourra N, Scriva A, Mansiaux Y, Gozlan S, Bennis M, Balaton A. Clusterin expression in gastrointestinal neuroendocrine tumours is highly correlated with location and is helpful in determining the origin of liver metastases. Histopathology. 2014; Nov. 65(5):642–50.
Article14. Tschopp J, Chonn A, Hertig S, French LE. Clusterin, the human apolipoprotein and complement inhibitor, binds to complement C7, C8 beta, and the b domain of C9. J Immunol. 1993; Aug. 151(4):2159–65.15. Song SY, Woo HJ, Bae CH, Kim YW, Kim YD. Expression of leptin receptor in nasal polyps: leptin as a mucosecretagogue. Laryngoscope. 2010; May. 120(5):1046–50.
Article16. Na HG, Bae CH, Choi YS, Song SY, Kim YD. Spleen tyrosine kinase induces MUC5AC expression in human airway epithelial cell. Am J Rhinol Allergy. 2016; Mar-Apr. 30(2):89–93.
Article17. Kim DH, Chu HS, Lee JY, Hwang SJ, Lee SH, Lee HM. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2004; Jun. 130(6):747–52.
Article18. Bae CH, Choi YS, Song SY, Kim YD. Effect of thymic stromal lymphopoietin on MUC5B expression in human airway epithelial cells. Biochem Biophys Res Commun. 2014; May. 448(2):231–5.19. Turner J, Jones CE. Regulation of mucin expression in respiratory diseases. Biochem Soc Trans. 2009; Aug. 37(Pt 4):877–81.
Article20. Woo HJ, Yoo WJ, Bae CH, Song SY, Kim YW, Park SY, et al. Leptin up-regulates MUC5B expression in human airway epithelial cells via mitogen-activated protein kinase pathway. Exp Lung Res. 2010; Jun. 36(5):262–9.
Article21. Essabbani A, Margottin-Goguet F, Chiocchia G. Identification of clusterin domain involved in NF-kappaB pathway regulation. J Biol Chem. 2010; Feb. 285(7):4273–7.22. Pucci S, Bonanno E, Pichiorri F, Angeloni C, Spagnoli LG. Modulation of different clusterin isoforms in human colon tumorigenesis. Oncogene. 2004; Mar. 23(13):2298–304.
Article23. Bhutia SK, Das SK, Kegelman TP, Azab B, Dash R, Su ZZ, et al. mda-7/IL-24 differentially regulates soluble and nuclear clusterin in prostate cancer. J Cell Physiol. 2012; May. 227(5):1805–13.
Article24. Jones SE, Jomary C. Clusterin. Int J Biochem Cell Biol. 2002; May. 34(5):427–31.
Article25. Trougakos IP, Djeu JY, Gonos ES, Boothman DA. Advances and challenges in basic and translational research on clusterin. Cancer Res. 2009; Jan. 69(2):403–6.
Article26. Falgarone G, Chiocchia G. Chapter 8. Clusterin: a multifacet protein at the crossroad of inflammation and autoimmunity. Adv Cancer Res. 2009; 104:139–70.27. Leskov KS, Klokov DY, Li J, Kinsella TJ, Boothman DA. Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem. 2003; Mar. 278(13):11590–600.
Article28. Garvin LM, Chen Y, Damsker JM, Rose MC. A novel dissociative steroid VBP15 reduces MUC5AC gene expression in airway epithelial cells but lacks the GRE mediated transcriptional properties of dexamethasone. Pulm Pharmacol Ther. 2016; Jun. 38:17–26.
Article29. Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NFkappaB paradigm. J Immunol. 2009; Nov. 183(10):6236–43.30. Kurakula K, Hamers AA, van Loenen P, de Vries CJ. 6-Mercaptopurine reduces cytokine and Muc5ac expression involving inhibition of NFkB activation in airway epithelial cells. Respir Res. 2015; Jun. 16:73.
Article31. Shim YJ, Kang BH, Jeon HS, Park IS, Lee KU, Lee IK, et al. Clusterin induces matrix metalloproteinase-9 expression via ERK1/2 and PI3K/Akt/NF-kB pathways in monocytes/macrophages. J Leukoc Biol. 2011; Oct. 90(4):761–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tussilagone suppressed the production and gene expression of MUC5AC mucin via regulating nuclear factor-kappa B signaling pathway in airway epithelial cells
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells
- Involvement of IKK/IkBα/NF-kB p65 Signaling into the Regulative Effect of Engeletin on MUC5AC Mucin Gene Expression in Human Airway Epithelial Cells
- Effect of Multi-Walled Carbon Nanotubes on MUC5AC and MUC5B Expression in Airway Epithelial Cells
- Triptolide Inhibits Lipopolysaccharide-Induced MUC5AC/5B Expression via Nuclear Factor-Kappa B in Human Airway Epithelial Cells