J Vet Sci.
2016 Dec;17(4):459-466. 10.4142/jvs.2016.17.4.459.
Physical-chemical and biological characterization of different preparations of equine chorionic gonadotropin
- Affiliations
-
- 1Agency for Agribusiness Technology of São Paulo, 13400-970 Piracicaba, Brazil. rherrera@apta.sp.gov.br
- 2Biotechnology Department, IPEN-CNEN, Cidade Universitária Sao Paulo, 05508-000 São Paulo, Brazil.
- KMID: 2412601
- DOI: http://doi.org/10.4142/jvs.2016.17.4.459
Abstract
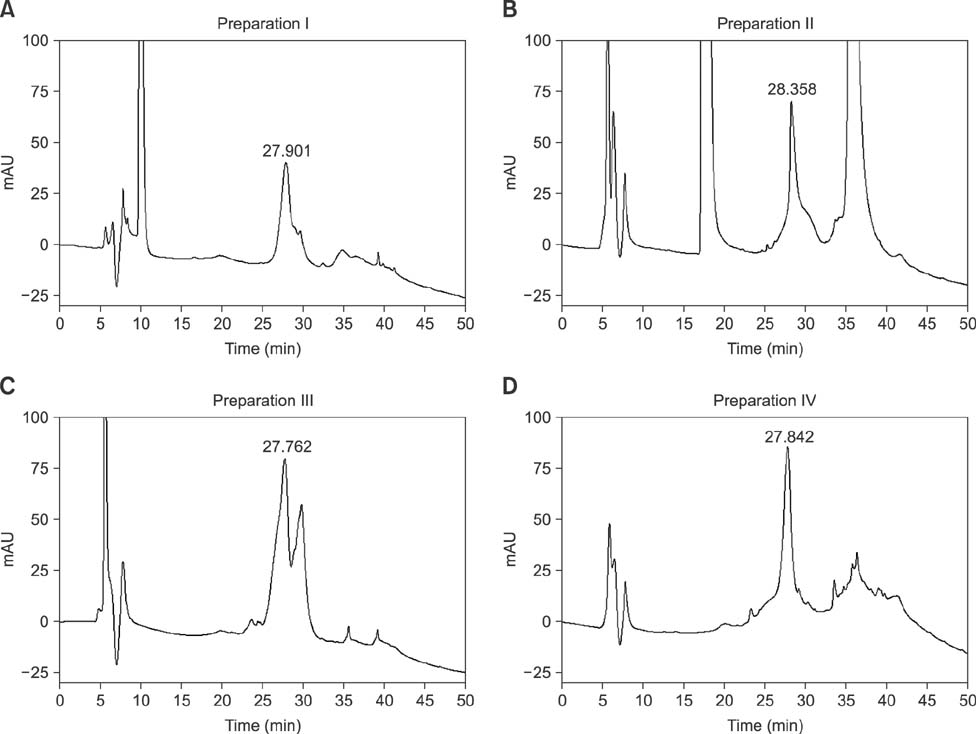
- Ovarian stimulation with commercial preparations of equine chorionic gonadotropin (eCG) produces extremely variable responses in domestic animals, ranging from excessive stimulation to practically no stimulation, when applied on the basis of their declared unitage. This study was conducted to analyze four commercial preparations from different manufacturers via reversed-phase HPLC (RP-HPLC) in comparison with a reference preparation and an official International Standard from the World Health Organization. The peaks obtained by this qualitative and quantitative physical-chemical analysis were compared using an in vivo bioassay based on the ovarian weight gain of prepubertal female rats. The RP-HPLC data showed one or two peaks close to a main peak (t(R) = 27.9 min), which were related to the in vivo bioactivity. Commercial preparations that have this altered peak showed very little or no in vivo activity, as demonstrated by rat ovarian weight and in peripubertal gilts induced to ovulate. Overall, these findings indicate that RP-HPLC can be a rapid and reliable tool to reveal changes in the physicochemical profile of commercial eCG that is apparently related to decreased biological activity of this hormone.
Keyword
MeSH Terms
Figure
Reference
-
1. Aggarwal BB, Farmer SW, Papkoff H, Seidel GE Jr. Biochemical properties of equine chorionic gonadotropin from two different pools of pregnant mare sera. Biol Reprod. 1980; 23:570–576.
Article2. Allen WR, Moor RM. The origin of the equine endometrial cups. I. Production of PMSG by fetal trophoblast cells. J Reprod Fertil. 1972; 29:313–316.3. Almeida BE, Oliveira JE, Carvalho CM, Dalmora SL, Bartolini P, Ribela MTCP. Analysis of human luteinizing hormone and human chorionic gonadotropin preparations of different origins by reversed-phase high-performance liquid chromatography. J Pharm Biomed Anal. 2010; 53:90–97.
Article4. Almeida BE, Oliveira JE, Damiani R, Dalmora SL, Bartolini P, Ribela MTCP. A pilot study on potency determination of human follicle-stimulating hormone: a comparison between reversed-phase high-performance liquid chromatography method and the in vivo bioassay. J Pharm Biomed Anal. 2011; 54:681–686.
Article5. Almeida BE, Oliveira JE, Damiani R, Dalmora SL, Bartolini P, Ribela MTCP. Qualitative and quantitative reversed-phase high performance liquid chromatographic analysis of glycoprotein hormones in the presence of a large excess of human serum albumin. J Pharm Biomed Anal. 2012; 63:160–164.
Article6. Baruselli PS, Reis EL, Marques MO, Nasser LF, Bó GA. The use of hormonal treatments to improve reproductive performance of anestrous beef cattle in tropical climates. Anim Reprod Sci. 2004; 82-83:479–486.
Article7. Birken S, Berger P, Bidart JM, Weber M, Bristow A, Norman R, Sturgeon K, Stenman UH. Preparation and characterization of new WHO reference reagents for human chorionic gonadotropin and metabolites. Clin Chem. 2003; 49:144–154.
Article8. Birken S, Maydelman Y, Gawinowicz MA. Preparation and analysis of the common urinary forms of human chorionic gonadotropin. Methods. 2000; 21:3–14.
Article9. Bó GA, Mapletoft RJ. Historical perspectives and recent research on superovulation in cattle. Theriogenology. 2014; 81:38–48.
Article10. Ciller UA, McFarlane JD, McFarlane JR. Commercial equine chorionic gonadotrophin isoform composition, immunoactivity, and bioactivity in a murine model using ovarian augmentation and testicular interstitial cell bioassays. Reprod Fertil Dev. 2009; 21:Suppl 9. 111–111.
Article11. Cole HH, Bigelow M, Finkel J, Rupp GR. Biological half-life of endogenous PMS following hysterectomy and studies on losses in urine and milk. Endocrinology. 1967; 81:927–930.
Article12. Cole HH, Erway J. 48-hour assay test for equine gonadotropin with results expressed in International Units. Endocrinology. 1941; 29:514–519.
Article13. Cole HH, Hart GH. The potency of blood serum of mares in progressive stages of pregnancy in effecting the sexual maturity of the immature rat. Am J Physiol. 1930; 93:57–68.
Article14. Forman RG, Marshall J, Robinson J, Cederholm Williams SA. A comparative analysis of two preparations of human chorionic gonadotrophin. Eur J Obstet Gynecol Reprod Biol. 1992; 46:123–127.
Article15. Gam LH, Tham SY, Latiff A. Immunoaffinity extraction and tandem mass spectrometric analysis of human chorionic gonadotropin in doping analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2003; 792:187–196.
Article16. Garthoff B, Hendriksen C, Bayol A, Goncalves D, Grauer A, de Leeuw R, van Noordwijk J, Pares M, Pirovano R, Rieth M, Ronneberger H, Spieser JM, Storring P, Vosbeck K, Weichert H. Safety and efficacy testing of hormones and related products. The report and recommendations of ECVAM Workshop 9. Altern Lab Anim. 1995; 23:699–712.
Article17. Gonzalez A, Wang H, Carruthers TD, Murphy BD, Mapletoft RJ. Superovulation in the cow with pregnant mare serum gonadotrophin: effects of dose and antipregnant mare serum gonadotrophin serum. Can Vet J. 1994; 35:158–162.18. Henderson KM, Weaver A, Wards RL, Lun S, McNatty KP. Comparison of commercial gonadotrophins using bioassays. Proc N Z Soc Anim Prod. 1990; 50:161–165.19. Huth JR, Norton SE, Lockridge O, Shikone T, Hsueh AJW, Ruddon RW. Bacterial expression and in vitro folding of the β-subunit of human chorionic gonadotropin (hCGβ) and functional assembly of recombinant hCGβ with hCGα. Endocrinology. 1994; 135:911–918.
Article20. Lecompte F, Roy F, Combarnous Y. International collaborative calibration of a preparation of equine chorionic gonadotrophin (eCG NZY-01) proposed as a new standard. J Reprod Fertil. 1998; 113:145–150.
Article21. Loureiro RF, de Oliveira JE, Torjesen PA, Bartolini P, Ribela MTCP. Analysis of intact human follicle-stimulating hormone preparations by reversed-phase high-performance liquid chromatography. J Chromatogr A. 2006; 1136:10–18.
Article22. Lunenfeld B. Historical perspectives in gonadotrophin therapy. Hum Reprod Update. 2004; 10:453–467.
Article23. Lustbader JW, Wu H, Birken S, Pollak S, Gawinowicz Kolks MA, Pound AM, Austen D, Hendrickson WA, Canfield RE. The expression, characterization, and crystallization of wild-type and selenomethionyl human chorionic gonadotropin. Endocrinology. 1995; 136:640–650.
Article24. Martinuk SD, Manning AW, Black WD, Murphy BD. Effects of carbohydrates on the pharmacokinetics and biological activity of equine chorionic gonadotrophin in vivo. Biol Reprod. 1991; 45:598–604.
Article25. McFarlane JR, Czekala NM, Papkoff H. Zebra chorionic gonadotropin: partial purification and characterization. Biol Reprod. 1991; 44:827–833.26. Murphy BD, Martinuk SD. Equine chorionic gonadotrophin. Endocr Rev. 1991; 12:27–44.27. Newcomb R, Christie WB, Rowson LEA, Walters DE, Bousfield WED. Influence of dose, repeated treatment and batch of hormone on ovarian response in heifers treated with PMSG. J Reprod Fertil. 1979; 56:113–118.
Article28. Remmele RL, Krishnan S, Callahan WJ. Development of stable lyophilized protein drug products. Curr Pharm Biotechnol. 2012; 13:471–496.
Article29. Ribela MTCP, Gout PW, de Oliveira JE, Bartolini P. HPLC analysis of human pituitary hormones for pharmaceutical applications. Curr Pharm Anal. 2006; 2:103–126.
Article30. Rostami B, Niasari-Naslaji A, Vojgani M, Nikjou D, Amanlou H, Gerami A. Effect of eCG on early resumption of ovarian activity in postpartum dairy cows. Anim Reprod Sci. 2011; 128:100–106.
Article31. Sá Filho MF, Torres-Júnior JRS, Penteado L, Gimenes LU, Ferreira RM, Ayres H, Castro E, Sales JNS, Baruselli PS. Equine chorionic gonadotropin improves the efficacy of a progestin-based fixed-time artificial insemination protocol in Nelore (Bos indicus) heifers. Anim Reprod Sci. 2010; 118:182–187.
Article32. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985; 150:76–86.
Article33. Souza AH, Viechnieski S, Lima FA, Silva FF, Araújo R, Bó GA, Wiltbank MC, Baruselli PS. Effects of equine chorionic gonadotropin and type of ovulatory stimulus in a timed-AI protocol on reproductive responses in dairy cows. Theriogenology. 2009; 72:10–21.
Article34. Wang W, Ignatius AA, Thakkar SV. Impact of residual impurities and contaminants on protein stability. J Pharm Sci. 2014; 103:1315–1330.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphometric and ultrastructural studies on the effects of human chorionic gonadotropin (hCG) in mouse uterus.
- The effect of human immunoglobulin and chorionic gonadotropin on the production of maternal blocking antibody
- Response of Human Chorionic Gonadotropin to 6-D-Trp-Gonadotropin-Releasing Hormone and Gonadotropin-Releasing Hormone Stimulation in the Culture Media of Normal Human Placenta of Different Gestational Ages
- Patterns of the decline in serum beta-human chorionic gonadotropin level in patients with tubal pregnancy following surgery by pelviscopy and by laparotomy
- The effect of the timing of human chorionic gonadotropin on in vitro fertilization