Ann Hepatobiliary Pancreat Surg.
2018 May;22(2):136-143. 10.14701/ahbps.2018.22.2.136.
Management of very late peritoneal metastasis of hepatocellular carcinoma 10 years after liver transplantation: Lessons from two cases
- Affiliations
-
- 1Division of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. shwang@amc.seoul.kr
- 2Multi Organ Transplant Center, King Fahad Specialist University Hospital, Dammam, Saudi Arabia.
- 3Department of Surgery, College of Medicine Inje University, Busan Paik Hospital, Busan, Korea.
- KMID: 2412428
- DOI: http://doi.org/10.14701/ahbps.2018.22.2.136
Abstract
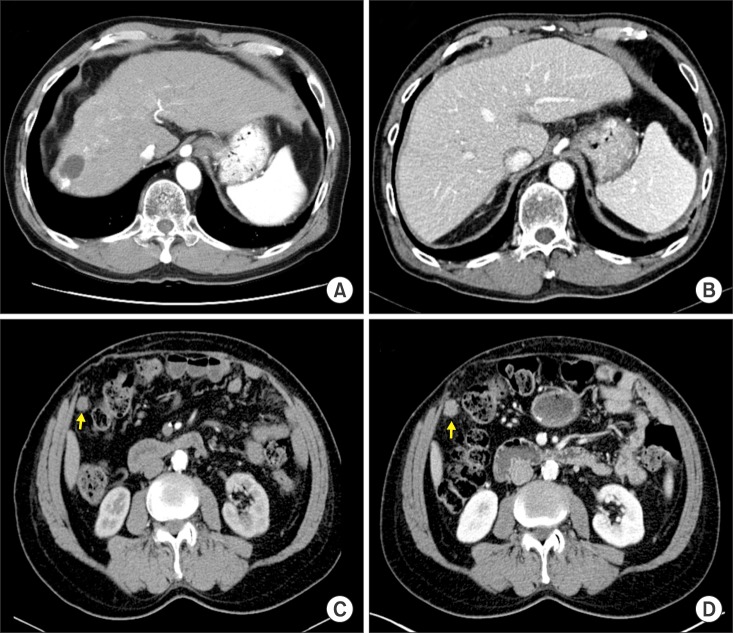
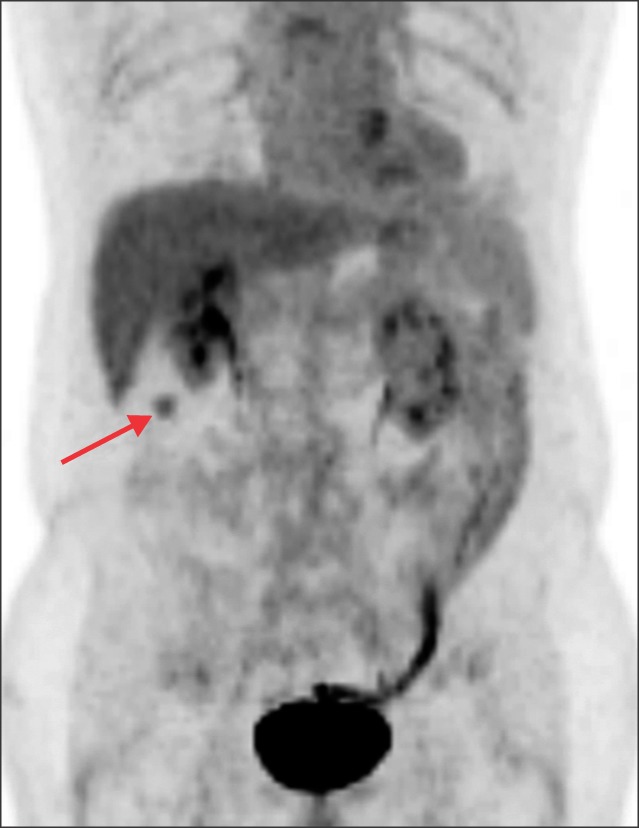
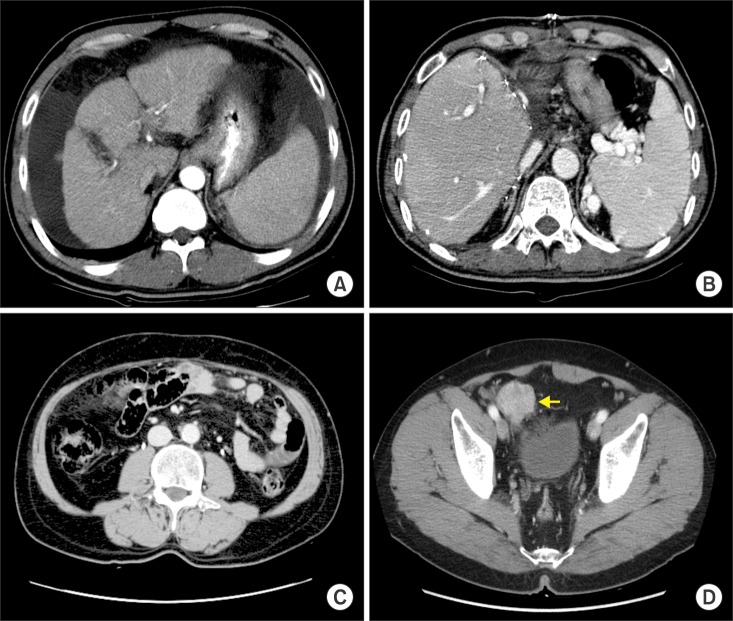
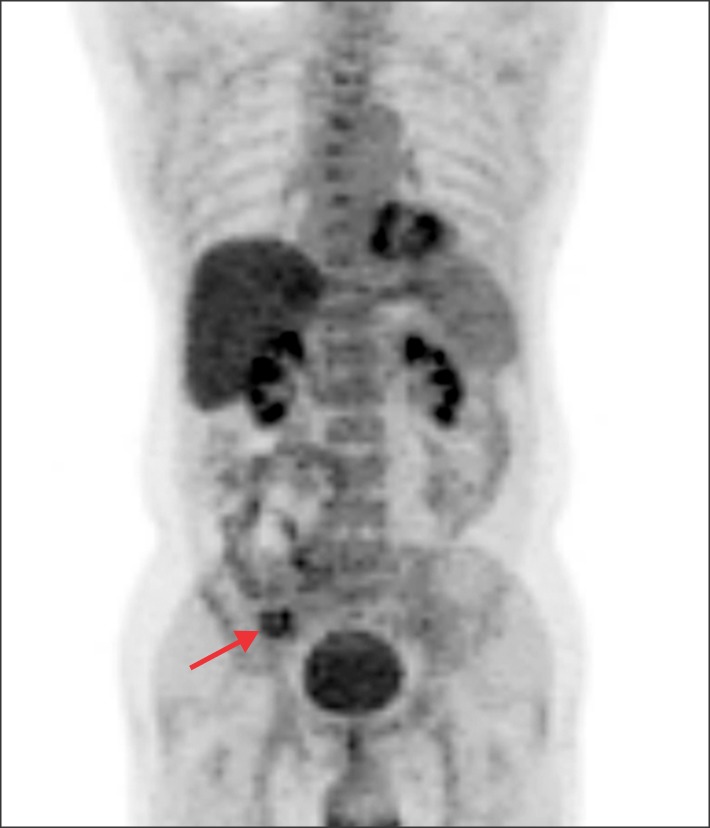
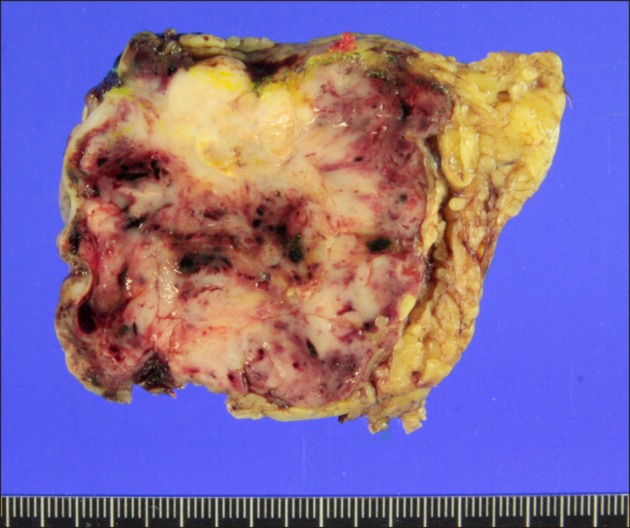
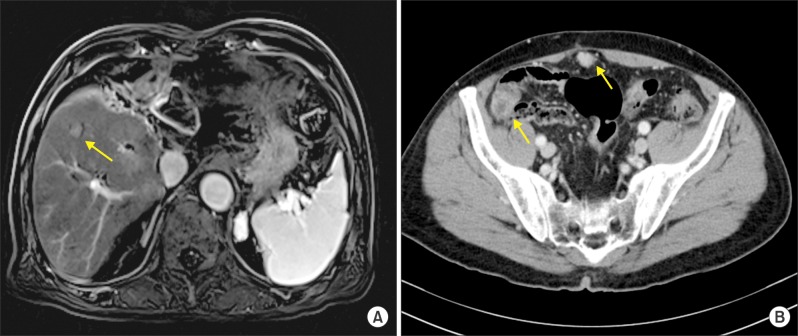
- Recurrence of hepatocellular carcinoma (HCC) 10 years after liver transplantation (LT) is very rare. Here, we present two cases of peritoneal metastasis of HCC that occurred 10 and 12 years after LT. A 77-year-old male who had undergone deceased-donor LT 10 years earlier showed slow progressive elevation of tumor marker levels over 6 months. Close observation with frequent imaging studies and monthly tumor marker analyses revealed a solitary peritoneal seeding mass. Imaging studies revealed that the mass was highly likely to be metastatic HCC. After excision of the mass, all tumor markers returned to the normal range. Over past 10 months, the patient has received everolimus monotherapy and half-dose sorafenib, and has shown no evidence of HCC recurrence. In the second case, marginally elevated tumor marker levels were detected in a 65-year-old male who had undergone living-donor LT 12 years earlier. After observation for 3 months, follow-up studies revealed a peritoneal seeding mass. Thorough imaging studies revealed that the mass was highly likely to be metastatic HCC. Two mass lesions were excised, and the patient was administered low-dose calcineruin inhibitor, sirolimus, and full-dose sorafenib. Subsequently, the tumor marker levels increased again and growth of new peritoneal seeding nodules was observed; therefore, sorafenib was stopped after 2 years of administration. During 6 years since HCC recurrence diagnosis, the patient has experienced slowly growing tumors, but has been doing well. For very late peritoneal metastasis of HCC, the therapeutic modalities include surgical resection if possible, everolimus monotherapy, and long-term use of sorafenib.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Cross-sectional analysis of immunosuppressive regimens focused on everolimus after liver transplantation in a Korean high-volume transplantation center
Sang-Hyun Kang, Shin Hwang, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Chul-Soo Ahn, Deok-Bog Moon, Ki-Hun Kim, Gil-Chun Park, Young-In Yoon, Yo-Han Park, Hui-Dong Cho, Jae-Hyun Kwon, Yong-Kyu Chung, Jin Uk Choi, Sung-Gyu Lee
Korean J Transplant. 2019;33(4):98-105. doi: 10.4285/jkstn.2019.33.4.98.
Reference
-
1. Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008; 14:935–945. PMID: 18581465.
Article2. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–699. PMID: 8594428.
Article3. Hwang S, Lee SG, Joh JW, Suh KS, Kim DG. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl. 2005; 11:1265–1272. PMID: 16184545.
Article4. Todo S, Furukawa H. Japanese Study Group on Organ Transplantation. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004; 240:451–459. PMID: 15319716.5. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001; 33:1394–1403. PMID: 11391528.6. Hwang S, Moon DB, Ahn CS, Kim KH, Ha TY, Song GW, et al. Risk-based long-term screening for hepatocellular carcinoma recurrence after living donor liver transplantation. Transplant Proc. 2013; 45:3076–3084. PMID: 24157040.
Article7. Park MS, Lee KW, Yi NJ, Choi YR, Kim H, Hong G, et al. Optimal tailored screening protocol after living donor liver transplantation for hepatocellular carcinoma. J Korean Med Sci. 2014; 29:1360–1366. PMID: 25368488.
Article8. Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010; 45:1272–1282. PMID: 20625772.
Article9. Taketomi A, Sanefuji K, Soejima Y, Yoshizumi T, Uhciyama H, Ikegami T, et al. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation. 2009; 87:531–537. PMID: 19307789.
Article10. Hwang S, Song GW, Lee YJ, Kim KH, Ahn CS, Moon DB, et al. Multiplication of tumor volume by two tumor markers is a post-resection prognostic predictor for solitary hepatocellular carcinoma. J Gastrointest Surg. 2016; 20:1807–1820. PMID: 27311982.
Article11. Yamashiki N, Sugawara Y, Tamura S, Tateishi R, Yoshida H, Kaneko J, et al. Postoperative surveillance with monthly serum tumor markers after living-donor liver transplantation for hepatocellular carcinoma. Hepatol Res. 2010; 40:278–286. PMID: 20070400.
Article12. Hwang S, Kim YH, Kim DK, Ahn CS, Moon DB, Kim KH, et al. Resection of pulmonary metastases from hepatocellular carcinoma following liver transplantation. World J Surg. 2012; 36:1592–1602. PMID: 22411088.
Article13. Ha TY, Hwang S, Ahn CS, Kim KH, Lee YJ, Moon DB, et al. Resection of metachronous adrenal metastasis after liver resection and transplantation for hepatocellular carcinoma. Dig Surg. 2014; 31:428–435. PMID: 25573138.
Article14. Hashimoto M, Sasaki K, Moriyama J, Matsuda M, Watanabe G. Resection of peritoneal metastases in patients with hepatocellular carcinoma. Surgery. 2013; 153:727–731. PMID: 22705249.
Article15. Kneuertz PJ, Cosgrove DP, Cameron AM, Kamel IR, Geschwind JF, Herman JM, et al. Multidisciplinary management of recurrent hepatocellular carcinoma following liver transplantation. J Gastrointest Surg. 2012; 16:874–881. PMID: 21975686.
Article16. Chua TC, Morris DL. Exploring the role of resection of extrahepatic metastases from hepatocellular carcinoma. Surg Oncol. 2012; 21:95–101. PMID: 21397495.
Article17. Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007; 67:369–391. PMID: 17335296.
Article18. Cholongitas E, Goulis I, Theocharidou E, Antoniadis N, Fouzas I, Giakoustidis D, et al. Everolimus-based immunosuppression in liver transplant recipients: a single-centre experience. Hepatol Int. 2014; 8:137–145. PMID: 26202415.
Article19. Jeng LB, Thorat A, Hsieh YW, Yang HR, Yeh CC, Chen TH, et al. Experience of using everolimus in the early stage of living donor liver transplantation. Transplant Proc. 2014; 46:744–748. PMID: 24767339.
Article20. Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014; 27:1039–1049. PMID: 24943720.
Article21. Kang SH, Hwang S, Ha TY, Song GW, Jung DH, Kim KH, et al. Tailored long-term immunosuppressive regimen for adult liver transplant recipients with hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg. 2014; 18:48–51. PMID: 26155248.
Article22. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004; 64:7099–7109. PMID: 15466206.
Article23. Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006; 98:326–334. PMID: 16507829.
Article24. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390. PMID: 18650514.
Article25. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34. PMID: 19095497.
Article26. Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015; 16:1344–1354. PMID: 26361969.
Article27. de'Angelis N, Landi F, Nencioni M, Palen A, Lahat E, Salloum C, et al. Role of sorafenib in patients with recurrent hepatocellular carcinoma after liver transplantation. Prog Transplant. 2016; 26:348–355. PMID: 27555074.28. Yoon DH, Ryoo BY, Ryu MH, Lee SG, Hwang S, Suh DJ, et al. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010; 40:768–773. PMID: 20494947.
Article29. Na GH, Hong TH, You YK, Kim DG. Clinical analysis of patients with hepatocellular carcinoma recurrence after living-donor liver transplantation. World J Gastroenterol. 2016; 22:5790–5799. PMID: 27433092.
Article30. Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, et al. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012; 18:45–52. PMID: 21932373.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Hepatocellular Carcinoma with Recurrent Peritoneal Metastasis after Hepatectomy Who Showed Complete Response by Surgical Resection
- Surgical Management of Hepatocellular Carcinoma
- Solitary Extrahepatic Intraabdominal Metastasis from Hepatocellular Carcinoma after Liver Transplantation
- Liver Transplantation for Hepatocellular Carcinoma
- Can hepatocellular carcinoma recurrence be prevented after liver transplantation?