J Vet Sci.
2018 May;19(3):434-445. 10.4142/jvs.2018.19.3.434.
Production of transgenic pigs using a pGFAP-CreER(T2)/EGFP(LoxP) inducible system for central nervous system disease models
- Affiliations
-
- 1Laboratory of Veterinary Embryology and Biotechnology, Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju 28644, Korea. shhyun@cbu.ac.kr
- 2Institute of Stem Cell & Regenerative Medicine, Chungbuk National University, Cheongju 28644, Korea.
- 3Department of Biotechnology, School of Life Sciences and Biotechnology, Korea University, Seoul 02841, Korea. hg-kim@korea.ac.kr
- KMID: 2412134
- DOI: http://doi.org/10.4142/jvs.2018.19.3.434
Abstract
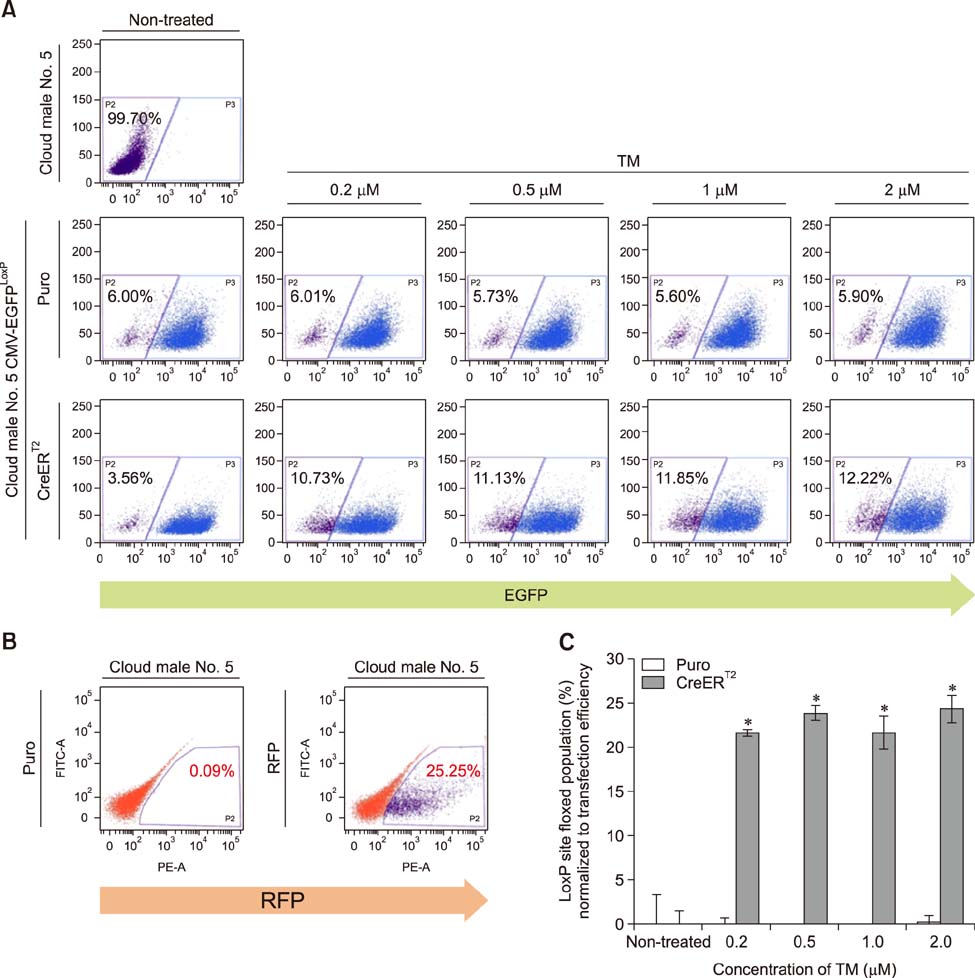
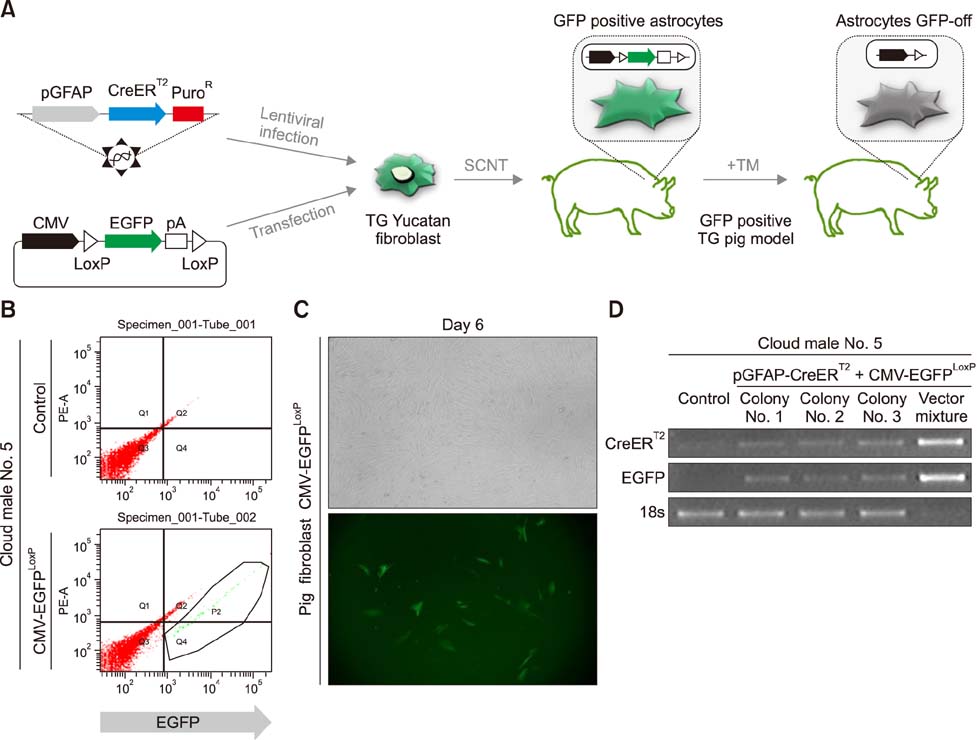
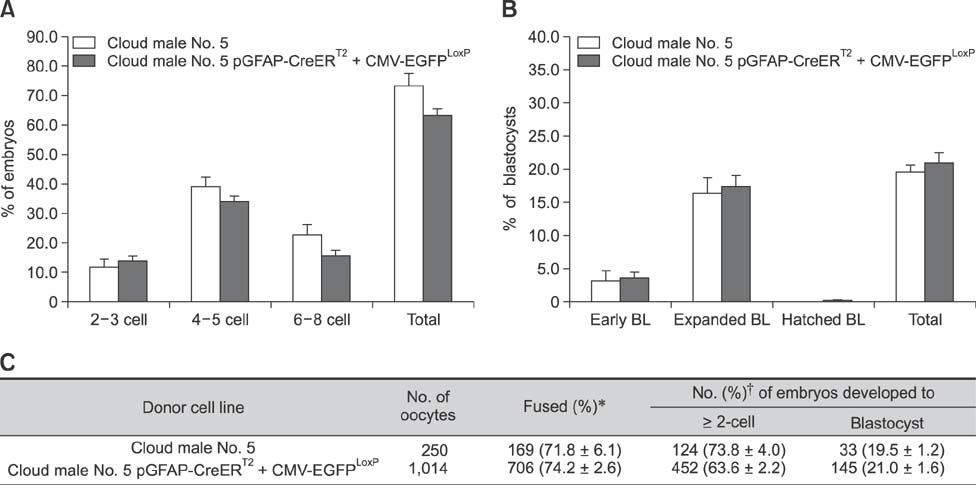
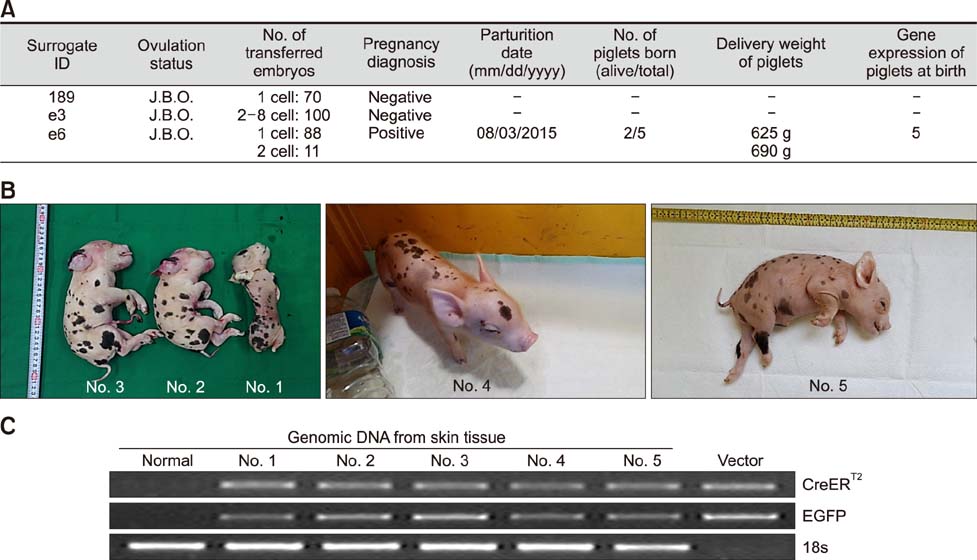
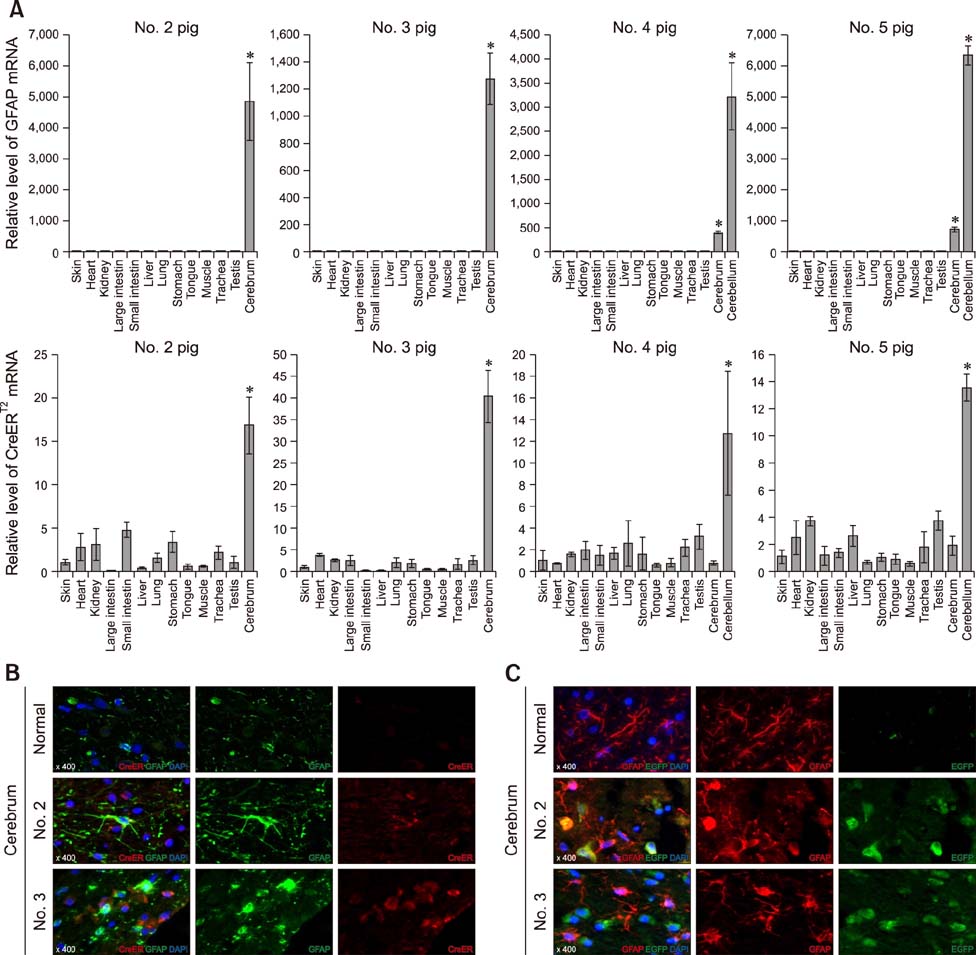
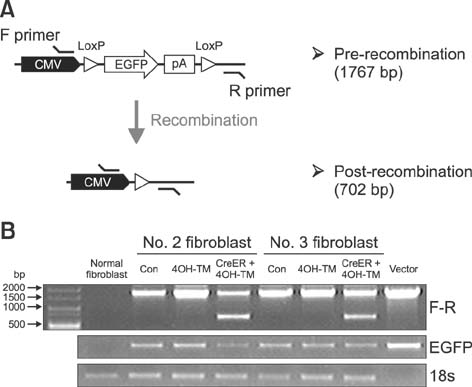
- Transgenic (TG) pigs are important in biomedical research and are used in disease modeling, pharmaceutical toxicity testing, and regenerative medicine. In this study, we constructed two vector systems by using the promoter of the pig glial fibrillary acidic protein (pGFAP) gene, which is an astrocyte cell marker. We established donor TG fibroblasts with pGFAP-CreER(T2)/LCMV-EGFP(LoxP) and evaluated the effect of the transgenes on TG-somatic cell nuclear transfer (SCNT) embryo development. Cleavage rates were not significantly different between control and transgene-donor groups. Embryo transfer was performed thrice just before ovulation of the surrogate sows. One sow delivered 5 TG piglets at 115 days after pregnancy. Polymerase chain reaction (PCR) analysis with genomic DNA isolated from skin tissues of TG pigs revealed that all 5 TG pigs had the transgenes. EGFP expression in all organs tested was confirmed by immunofluorescence staining and PCR. Real-time PCR analysis showed that pGFAP promoter-driven Cre fused to the mutated human ligand-binding domain of the estrogen receptor (CreER(T2)) mRNA was highly expressed in the cerebrum. Semi-nested PCR analysis revealed that CreER(T2)-mediated recombination was induced in cerebrum and cerebellum but not in skin. Thus, we successfully generated a TG pig with a 4-hydroxytamoxifen (TM)-inducible pGFAP-CreER(T2)/EGFP(LoxP) recombination system via SCNT.
Keyword
MeSH Terms
-
Animals, Genetically Modified
Astrocytes
Central Nervous System*
Cerebellum
Cerebrum
DNA
Embryo Transfer
Embryonic Development
Estrogens
Female
Fibroblasts
Fluorescent Antibody Technique
Glial Fibrillary Acidic Protein
Humans
Nuclear Transfer Techniques
Ovulation
Polymerase Chain Reaction
Pregnancy
Real-Time Polymerase Chain Reaction
Recombination, Genetic
Regenerative Medicine
RNA, Messenger
Skin
Swine*
Tissue Donors
Toxicity Tests
Transgenes
DNA
Estrogens
Glial Fibrillary Acidic Protein
RNA, Messenger
Figure
Reference
-
1. Bradford J, Shin JY, Roberts M, Wang CE, Li XJ, Li S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc Natl Acad Sci U S A. 2009; 106:22480–22485.
Article2. Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994; 14:1030–1037.
Article3. Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998; 8:1323–1326.
Article4. Daschil N, Humpel C. Green-fluorescent protein+ astrocytes attach to beta-amyloid plaques in an Alzheimer mouse model and are sensitive for clasmatodendrosis. Front Aging Neurosci. 2016; 8:75.
Article5. de Blas AL. Monoclonal antibodies to specific astroglial and neuronal antigens reveal the cytoarchitecture of the Bergmann glia fibers in the cerebellum. J Neurosci. 1984; 4:265–273.
Article6. Du Y, Kragh PM, Zhang Y, Li J, Schmidt M, Bøgh IB, Zhang X, Purup S, Jørgensen AL, Pedersen AM, Villemoes K, Yang H, Bolund L, Vajta G. Piglets born from handmade cloning, an innovative cloning method without micromanipulation. Theriogenology. 2007; 68:1104–1110.
Article7. Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985; 8:203–214.
Article8. Eun K, Hwang SU, Jeon HM, Hyun SH, Kim H. Comparative analysis of human, mouse, and pig glial fibrillary acidic protein gene structures. Anim Biotechnol. 2016; 27:126–132.
Article9. Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A. 1996; 93:10887–10890.
Article10. Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997; 237:752–757.
Article11. Feil S, Valtcheva N, Feil R. Inducible Cre mice. Methods Mol Biol. 2009; 530:343–363.
Article12. Grindley ND, Whiteson KL, Rice PA. Mechanisms of site-specific recombination. Annu Rev Biochem. 2006; 75:567–605.
Article13. Herbst F, Ball CR, Tuorto F, Nowrouzi A, Wang W, Zavidij O, Dieter SM, Fessler S, van der Hoeven F, Kloz U, Lyko F, Schmidt M, von Kalle C, Glimm H. Extensive methylation of promoter sequences silences lentiviral transgene expression during stem cell differentiation in vivo. Mol Ther. 2012; 20:1014–1021.
Article14. Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009; 3:31.
Article15. Holm IE, Alstrup AK, Luo Y. Genetically modified pig models for neurodegenerative disorders. J Pathol. 2016; 238:267–287.
Article16. Howells DW, Porritt MJ, Rewell SS, O'Collins V, Sena ES, van der Worp HB, Traystman RJ, Macleod MR. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010; 30:1412–1431.
Article17. Huszthy PC, Daphu I, Niclou SP, Stieber D, Nigro JM, Sakariassen PØ, Miletic H, Thorsen F, Bjerkvig R. In vivo models of primary brain tumors: pitfalls and perspectives. Neuro Oncol. 2012; 14:979–993.
Article18. Hwang SU, Jeon Y, Yoon JD, Cai L, Kim E, Yoo H, Kim KJ, Park KM, Jin M, Kim H, Hyun SH. Effect of ganglioside GT1b on the in vitro maturation of porcine oocytes and embryonic development. J Reprod Dev. 2015; 61:549–557.
Article19. Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ERT and Cre-ERT2 recombinases. Nucleic Acids Res. 1999; 27:4324–4327.
Article20. Jin YX, Jeon Y, Lee SH, Kwon MS, Kim T, Cui XS, Hyun SH, Kim NH. Production of pigs expressing a transgene under the control of a tetracycline-inducible system. PLoS One. 2014; 9:e86146.
Article21. Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995; 16:351–380.
Article22. Kim CS, Choi SJ, Park CY, Li C, Choi JS. Effects of silybinin on the pharmacokinetics of tamoxifen and its active metabolite, 4-hydroxytamoxifen in rats. Anticancer Res. 2010; 30:79–85.23. King TJ, Dobrinsky JR, Zhu J, Finlayson HA, Bosma W, Harkness L, Ritchie WA, Travers A, McCorquodale C, Day BN, Dinnyés A, De Sousa PA, Wilmut I. Embryo development and establishment of pregnancy after embryo transfer in pigs: coping with limitations in the availability of viable embryos. Reproduction. 2002; 123:507–515.
Article24. Kong Q, Wu M, Huan Y, Zhang L, Liu H, Bou G, Luo Y, Mu Y, Liu Z. Transgene expression is associated with copy number and cytomegalovirus promoter methylation in transgenic pigs. PLoS One. 2009; 4:e6679.
Article25. Kues WA, Schwinzer R, Wirth D, Verhoeyen E, Lemme E, Herrmann D, Barg-Kues B, Hauser H, Wonigeit K, Niemann H. Epigenetic silencing and tissue independent expression of a novel tetracycline inducible system in double-transgenic pigs. FASEB J. 2006; 20:1200–1202.
Article26. Le Y, Sauer B. Conditional gene knockout using Cre recombinase. Mol Biotechnol. 2001; 17:269–275.
Article27. Levis R, Hazelrigg T, Rubin GM. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science. 1985; 229:558–561.
Article28. Li S, Flisikowska T, Kurome M, Zakhartchenko V, Kessler B, Saur D, Kind A, Wolf E, Flisikowski K, Schnieke A. Dual fluorescent reporter pig for Cre recombination: transgene placement at the ROSA26 locus. PLoS One. 2014; 9:e102455.29. Li X, Yang Y, Bu L, Guo X, Tang C, Song J, Fan N, Zhao B, Ouyang Z, Liu Z, Zhao Y, Yi X, Quan L, Liu S, Yang Z, Ouyang H, Chen YE, Wang Z, Lai L. Rosa26-targeted swine models for stable gene over-expression and Cre-mediated lineage tracing. Cell Res. 2014; 24:501–504.
Article30. Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995; 92:6991–6995.
Article31. Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000; 26:99–109.
Article32. Niemann H, Tian XC, King WA, Lee RS. Epigenetic reprogramming in embryonic and foetal development upon somatic cell nuclear transfer cloning. Reproduction. 2008; 135:151–163.
Article33. No D, Yao TP, Evans RM. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci U S A. 1996; 93:3346–3351.
Article34. Ogura A, Inoue K, Wakayama T. Recent advancements in cloning by somatic cell nuclear transfer. Philos Trans R Soc Lond B Biol Sci. 2013; 368:20110329.
Article35. Orian JM, Lee CS, Weiss LM, Brandon MR. The expression of a metallothionein-ovine growth hormone fusion gene in transgenic mice does not impair fertility but results in pathological lesions in the liver. Endocrinology. 1989; 124:455–463.
Article36. Schuurman HJ, Pierson RN 3rd. Progress towards clinical xenotransplantation. Front Biosci. 2008; 13:204–220.
Article37. Uchida M, Shimatsu Y, Onoe K, Matsuyama N, Niki R, Ikeda JE, Imai H. Production of transgenic miniature pigs by pronuclear microinjection. Transgenic Res. 2001; 10:577–582.38. Walker SC, Shin T, Zaunbrecher GM, Romano JE, Johnson GA, Bazer FW, Piedrahita JA. A highly efficient method for porcine cloning by nuclear transfer using in vitro-matured oocytes. Cloning Stem Cells. 2002; 4:105–112.
Article39. Young LE, Sinclair KD, Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod. 1998; 3:155–163.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Functional Study of Gene using Inducible Cre System
- Generation of Astrocyte-Specific MAOB Conditional Knockout Mouse with Minimal Tonic GABA Inhibition
- The value of computerized axial tomography of the brain in children with central nervous system disorders
- Treatment of pediatric central nervous system infections
- The Role of a Neurovascular Signaling Pathway Involving Hypoxia-Inducible Factor and Notch in the Function of the Central Nervous System