J Vet Sci.
2018 May;19(3):368-374. 10.4142/jvs.2018.19.3.368.
Prevalence, toxin gene profile, antibiotic resistance, and molecular characterization of Clostridium perfringens from diarrheic and non-diarrheic dogs in Korea
- Affiliations
-
- 1Division of Microbiology, National Center for Toxicological Research, U.S. Food and Drug Administration, Jefferson, AR 72079, USA. Kidon.Sung@fda.hhs.gov
- 2KU Center for One Health, College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea.
- 3Joy Animal Hospital, Ansan 15388, Korea.
- 4Veterinary Clinical Pathology, College of Veterinary Medicine, Chonbuk National University, Jeonju 54896, Korea.
- KMID: 2412127
- DOI: http://doi.org/10.4142/jvs.2018.19.3.368
Abstract
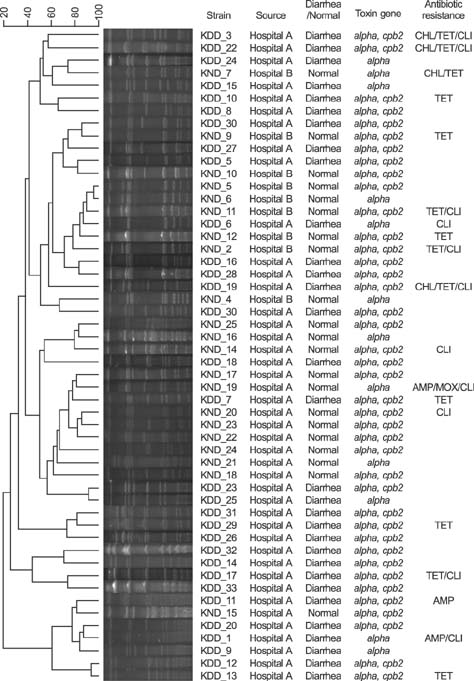
- Clostridium perfringens causes diarrhea and other diseases in animals and humans. We investigated the prevalence, toxin gene profiles, and antibiotic resistance of C. perfringens isolated from diarrheic dogs (DD) and non-diarrheic dogs (ND) in two animal hospitals in Seoul, Korea. Fecal samples were collected from clinically DD (n = 49) and ND (n = 34). C. perfringens was isolated from 31 of 49 DD (63.3%) and 21 of 34 ND dogs (61.8%). All C. perfringens strains were positive for the α toxin gene, but not for the β, ε, or ι toxin genes; therefore, all strains were identified as type A C. perfringens. All isolates were cpe-negative, whereas the β2 toxin gene was identified in 83.9% and 61.9% of isolates from DD and ND, respectively. Most isolates were susceptible to ampicillin (94%), chloramphenicol (92%), metronidazole (100%), moxifloxacin (96%), and imipenem (100%). However, 25.0% and 21.2% of isolates were resistant to tetracycline and clindamycin, respectively. Molecular subtyping of the isolated strains was performed by using pulsed-field gel electrophoresis. Fifty-two isolates were classified into 48 pulsotypes based on more than 90% similarity of banding patterns. No notable differences were observed among the isolates from DD and ND.
Keyword
MeSH Terms
-
Ampicillin
Animals
Bacterial Toxins
Chloramphenicol
Clindamycin
Clostridium perfringens*
Clostridium*
Diarrhea
Dogs*
Drug Resistance
Drug Resistance, Microbial*
Electrophoresis, Gel, Pulsed-Field
Hospitals, Animal
Humans
Imipenem
Korea*
Metronidazole
Prevalence*
Seoul
Tetracycline
Ampicillin
Bacterial Toxins
Chloramphenicol
Clindamycin
Imipenem
Metronidazole
Tetracycline
Figure
Reference
-
1. Baums CG, Schotte U, Amtsberg G, Goethe R. Diagnostic multiplex PCR for toxin genotyping of Clostridium perfringens isolates. Vet Microbiol. 2004; 100:11–16.
Article2. Berset-Istratescu CM, Glardon OJ, Magouras I, Frey CF, Gobeli S, Burgener IA. Follow-up of 100 dogs with acute diarrhoea in a primary care practice. Vet J. 2014; 199:188–190.
Article3. Bueschel DM, Jost BH, Billington SJ, Trinh HT, Songer JG. Prevalence of cpb2, encoding beta2 toxin, in Clostridium perfringens field isolates: correlation of genotype with phenotype. Vet Microbiol. 2003; 94:121–129.
Article4. Chon JW, Hyeon JY, Park JH, Song KY, Kim JH, Seo KH. Improvement of mannitol-yolk-polymyxin B agar by supplementing with trimethoprim for quantitative detection of Bacillus cereus in foods. J Food Prot. 2012; 75:1342–1345.
Article5. Chon JW, Park JS, Hyeon JY, Park C, Song KY, Hong KW, Hwang IG, Kwak HS, Seo KH. Development of real-time PCR for the detection of Clostridium perfringens in meats and vegetables. J Microbiol Biotechnol. 2012; 22:530–534.
Article6. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI document M100-S24. Wayne: CLSI;2014.7. Dornbusch K, Nord CE, Dahlbäck A. Antibiotic susceptibility of Clostridium species isolated from human infections. Scand J Infect Dis. 1975; 7:127–134.
Article8. Dutta GN, Devriese LA. Macrolide-lincosamide-streptogramin resistance patterns in Clostridium perfringens from animals. Antimicrob Agents Chemother. 1981; 19:274–278.
Article9. Goldstein MR, Kruth SA, Bersenas AM, Holowaychuk MK, Weese JS. Detection and characterization of Clostridium perfringens in the feces of healthy and diarrheic dogs. Can J Vet Res. 2012; 76:161–165.10. Hackett T, Lappin MR. Prevalence of enteric pathogens in dogs of north-central Colorado. J Am Anim Hosp Assoc. 2003; 39:52–56.
Article11. Herholz C, Miserez R, Nicolet J, Frey J, Popoff M, Gibert M, Gerber H, Straub R. Prevalence of β2-toxigenic Clostridium perfringens in horses with intestinal disorders. J Clin Microbiol. 1999; 37:358–361.
Article12. Kather EJ, Marks SL, Foley JE. Determination of the prevalence of antimicrobial resistance genes in canine Clostridium perfringens isolates. Vet Microbiol. 2006; 113:97–101.
Article13. Klaasen HL, Molkenboer MJ, Bakker J, Miserez R, Häni H, Frey J, Popoff MR, van den Bosch JF. Detection of the β2 toxin gene of Clostridium perfringens in diarrhoeic piglets in the Netherlands and Switzerland. FEMS Immunol Med Microbiol. 1999; 24:325–332.
Article14. Marks SL, Kather EJ. Antimicrobial susceptibilities of canine Clostridium difficile and Clostridium perfringens isolates to commonly utilized antimicrobial drugs. Vet Microbiol. 2003; 94:39–45.
Article15. Marks SL, Kather EJ, Kass PH, Melli AC. Genotypic and phenotypic characterization of Clostridium perfringens and Clostridium difficile in diarrheic and healthy dogs. J Vet Intern Med. 2002; 16:533–540.
Article16. Marks SL, Rankin SC, Byrne BA, Weese JS. Enteropathogenic bacteria in dogs and cats: diagnosis, epidemiology, treatment, and control. J Vet Intern Med. 2011; 25:1195–1208.
Article17. Márquez-González M, Cabrera-Díaz E, Hardin MD, Harris KB, Lucia LM, Castillo A. Survival and germination of Clostridium perfringens spores during heating and cooling of ground pork. J Food Prot. 2012; 75:682–689.18. Matushek MG, Bonten MJ, Hayden MK. Rapid preparation of bacterial DNA for pulsed-field gel electrophoresis. J Clin Microbiol. 1996; 34:2598–2600.
Article19. McDermott PF, Walker RD, White DG. Antimicrobials: modes of action and mechanisms of resistance. Int J Toxicol. 2003; 22:135–143.
Article20. McKenzie E, Riehl J, Banse H, Kass PH, Nelson S Jr, Marks SL. Prevalence of diarrhea and enteropathogens in racing sled dogs. J Vet Intern Med. 2010; 24:97–103.
Article21. Meer RR, Songer JG. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am J Vet Res. 1997; 58:702–705.22. Minamoto Y, Dhanani N, Markel ME, Steiner JM, Suchodolski JS. Prevalence of Clostridium perfringens, Clostridium perfringens enterotoxin and dysbiosis in fecal samples of dogs with diarrhea. Vet Microbiol. 2014; 174:463–473.
Article23. Nauerby B, Pedersen K, Madsen M. Analysis by pulsed-field gel electrophoresis of the genetic diversity among Clostridium perfringens isolates from chickens. Vet Microbiol. 2003; 94:257–266.
Article24. Park M, Deck J, Foley SL, Nayak R, Songer JG, Seibel JR, Khan SA, Rooney AP, Hecht DW, Rafii F. Diversity of Clostridium perfringens isolates from various sources and prevalence of conjugative plasmids. Anaerobe. 2016; 38:25–35.
Article25. Rood JI, Maher EA, Somers EB, Campos E, Duncan CL. Isolation and characterization of multiply antibiotic-resistant Clostridium perfringens strains from porcine feces. Antimicrob Agents Chemother. 1978; 13:871–880.
Article26. Salari Sedigh H, Rajabioun M, Razmyar J, Kazemi Mehrjerdi H. An unusual necrotic myositis by Clostridium perfringens in a German Shepherd dog: a clinical report, bacteriological and molecular identification. Vet Res Forum. 2015; 6:349–353.27. Schentag JJ, Gilliland KK, Paladino JA. What have we learned from pharmacokinetic and pharmacodynamic theories? Clin Infect Dis. 2001; 32:Suppl 1. S39–S46.
Article28. Silva RO, Lobato FC. Clostridium perfringens: a review of enteric diseases in dogs, cats and wild animals. Anaerobe. 2015; 33:14–17.
Article29. Silva RO, Santos RL, Pires PS, Pereira LC, Pereira ST, Duarte MC, de Assis RA, Lobato FC. Detection of toxins A/B and isolation of Clostridium difficile and Clostridium perfringens from dogs in Minas Gerais, Brazil. Braz J Microbiol. 2013; 44:133–137.
Article30. Songer JG, Uzal FA. Clostridial enteric infections in pigs. J Vet Diagn Invest. 2005; 17:528–536.
Article31. Thiede S, Goethe R, Amtsberg G. Prevalence of β2 toxin gene of Clostridium perfringens type A from diarrhoeic dogs. Vet Rec. 2001; 149:273–274.
Article32. Tupler T, Levy JK, Sabshin SJ, Tucker SJ, Greiner EC, Leutenegger CM. Enteropathogens identified in dogs entering a Florida animal shelter with normal feces or diarrhea. J Am Vet Med Assoc. 2012; 241:338–343.
Article33. Uzal FA, Vidal JE, McClane BA, Gurjar AA. Clostridium perfringens toxins involved in mammalian veterinary diseases. Open Toxinology J. 2010; 2:24–42.34. Weese JS, Staempfli HR, Prescott JF, Kruth SA, Greenwood SJ, Weese HE. The roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in diarrhea in dogs. J Vet Intern Med. 2001; 15:374–378.
Article35. Zerbini L, Ossiprandi MC. Molecular typing of Clostridium perfringens strains isolated from dogs by toxin gene amplification. Ann Fac Med Vet Di Parma. 2009; 29:115–128.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial resistance and frequency of BlaTEM in Escherichia coli isolated from non-diarrheic and diarrheic piglets
- Prevalence of Cryptosporidium sp. infection in diarrheic and non-diarrheic humans in Iran
- Isolation of Enterotoxin - positive Strains of Clostridium perfringens Type A in Korea
- Molecular Epidemiology of Clostridium perfringens Isolated from Food Poisoning in Seoul, 2013
- Prevalence and Molecular Characterization of Intestinal Trichomonads in Pet Dogs in East China