Korean J Radiol.
2018 Jun;19(3):443-451. 10.3348/kjr.2018.19.3.443.
Utility of Readout-Segmented Echo-Planar Imaging-Based Diffusion Kurtosis Imaging for Differentiating Malignant from Benign Masses in Head and Neck Region
- Affiliations
-
- 1Department of Radiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China. wfy_njmu@163.com
- KMID: 2410815
- DOI: http://doi.org/10.3348/kjr.2018.19.3.443
Abstract
OBJECTIVE
To compare the diagnostic performance of readout-segmented echo-planar imaging (RS-EPI)-based diffusion kurtosis imaging (DKI) and that of diffusion-weighted imaging (DWI) for differentiating malignant from benign masses in head and neck region.
MATERIALS AND METHODS
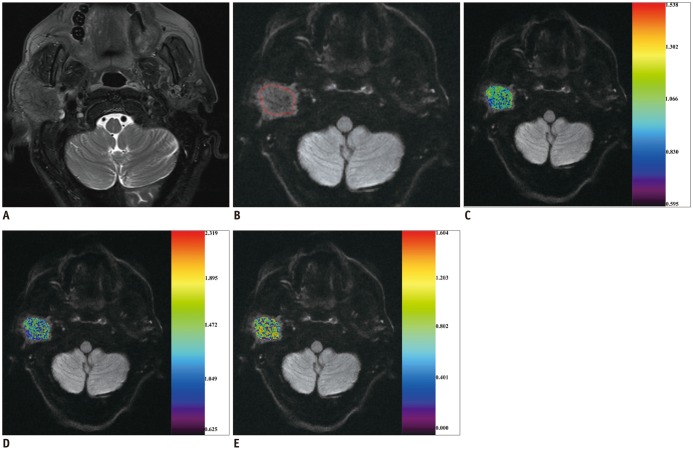
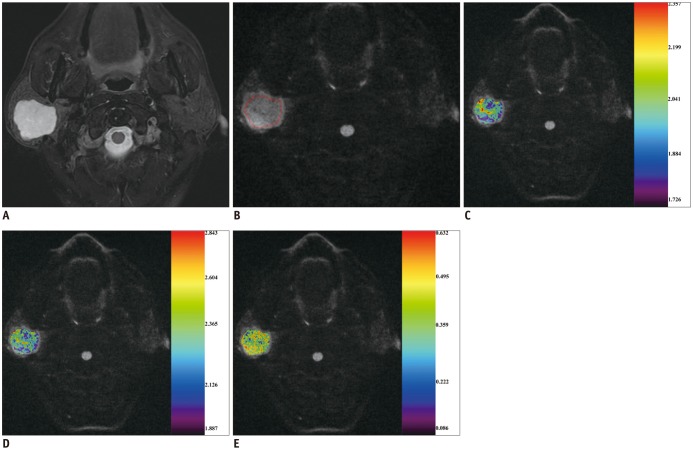
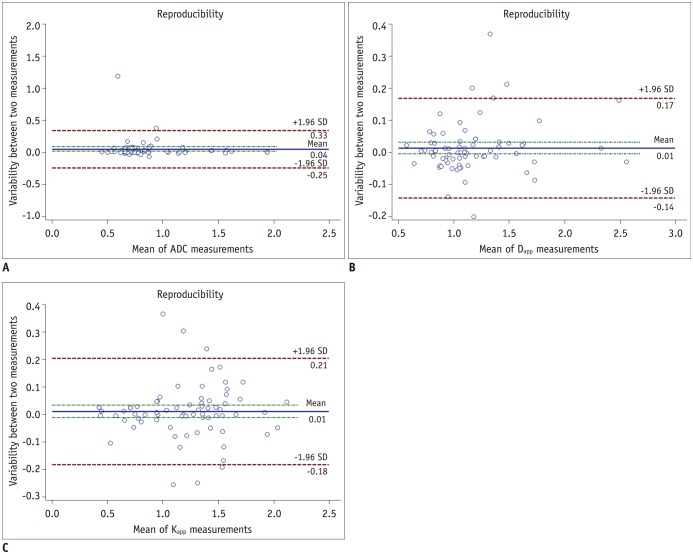
Between December 2014 and April 2016, we retrospectively enrolled 72 consecutive patients with head and neck masses who had undergone RS-EPI-based DKI scan (b value of 0, 500, 1000, and 1500 s/mm2) for pretreatment evaluation. Imaging data were post-processed by using monoexponential and diffusion kurtosis (DK) model for quantitation of apparent diffusion coefficient (ADC), apparent diffusion for Gaussian distribution (Dapp), and apparent kurtosis coefficient (Kapp). Unpaired t test and Mann-Whitney U test were used to compare differences of quantitative parameters between malignant and benign groups. Receiver operating characteristic curve analyses were performed to determine and compare the diagnostic ability of quantitative parameters in predicting malignancy.
RESULTS
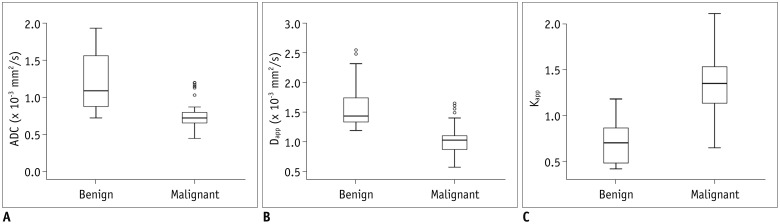
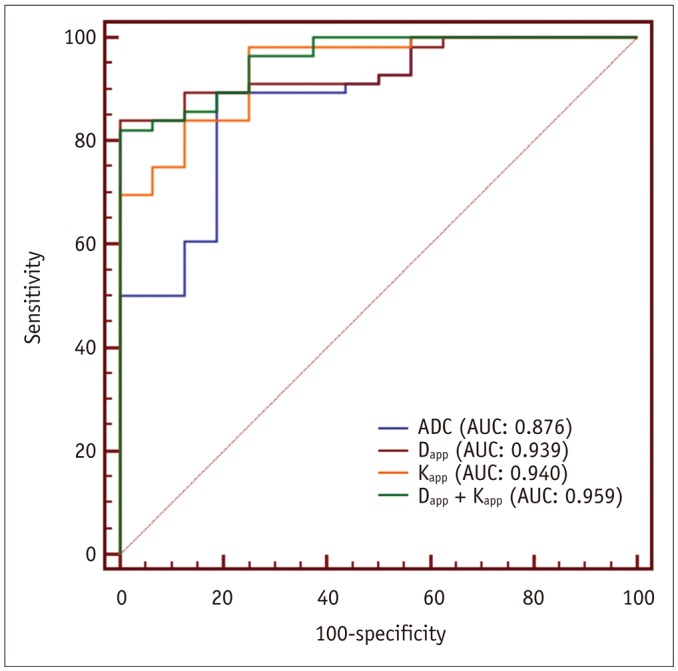
Malignant group demonstrated significantly lower ADC (0.754 ± 0.167 vs. 1.222 ± 0.420, p < 0.001) and Dapp (1.029 ± 0.226 vs. 1.640 ± 0.445, p < 0.001) while higher Kapp (1.344 ± 0.309 vs. 0.715 ± 0.249, p < 0.001) than benign group. Using a combination of Dapp and Kapp as diagnostic index, significantly better differentiating performance was achieved than using ADC alone (area under curve: 0.956 vs. 0.876, p = 0.042).
CONCLUSION
Compared to DWI, DKI could provide additional data related to tumor heterogeneity with significantly better differentiating performance. Its derived quantitative metrics could serve as a promising imaging biomarker for differentiating malignant from benign masses in head and neck region.
Keyword
MeSH Terms
Figure
Reference
-
1. Lewis-Jones H, Colley S, Gibson D. Imaging in head and neck cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016; 130:S28–S31. PMID: 27841111.
Article2. Wippold FJ 2nd. Head and neck imaging: the role of CT and MRI. J Magn Reson Imaging. 2007; 25:453–465. PMID: 17279529.
Article3. Serifoğlu Ì, Oz ÌÌ, Damar M, Tokgöz Ö, Yazgan Ö, Erdem Z. Diffusion-weighted imaging in the head and neck region: usefulness of apparent diffusion coefficient values for characterization of lesions. Diagn Interv Radiol. 2015; 21:208–214. PMID: 25910284.
Article4. Schafer J, Srinivasan A, Mukherji S. Diffusion magnetic resonance imaging in the head and neck. Magn Reson Imaging Clin N Am. 2011; 19:55–67. PMID: 21129635.
Article5. Srinivasan A, Dvorak R, Perni K, Rohrer S, Mukherji SK. Differentiation of benign and malignant pathology in the head and neck using 3T apparent diffusion coefficient values: early experience. AJNR Am J Neuroradiol. 2008; 29:40–44. PMID: 17921228.
Article6. Ginat DT, Mangla R, Yeaney G, Johnson M, Ekholm S. Diffusion-weighted imaging for differentiating benign from malignant skull lesions and correlation with cell density. AJR Am J Roentgenol. 2012; 198:W597–W601. PMID: 22623576.
Article7. Thoeny HC, De Keyzer F, King AD. Diffusion-weighted MR imaging in the head and neck. Radiology. 2012; 263:19–32. PMID: 22438440.
Article8. Xu XQ, Hu H, Su GY, Liu H, Hong XN, Shi HB, et al. Utility of histogram analysis of ADC maps for differentiating orbital tumors. Diagn Interv Radiol. 2016; 22:161–167. PMID: 26829400.
Article9. Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010; 23:698–710. PMID: 20632416.
Article10. Sun K, Chen X, Chai W, Fei X, Fu C, Yan X, et al. Breast cancer: diffusion kurtosis MR imaging-diagnostic accuracy and correlation with clinical-pathologic factors. Radiology. 2015; 277:46–55. PMID: 25938679.
Article11. Nogueira L, Brandão S, Matos E, Nunes RG, Loureiro J, Ramos I, et al. Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol. 2014; 24:1197–1203. PMID: 24658871.
Article12. Rosenkrantz AB, Sigmund EE, Winnick A, Niver BE, Spieler B, Morgan GR, et al. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants. Magn Reson Imaging. 2012; 30:1534–1540. PMID: 22819175.
Article13. Rosenkrantz AB, Sigmund EE, Johnson G, Babb JS, Mussi TC, Melamed J, et al. Prostate cancer: feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology. 2012; 264:126–135. PMID: 22550312.
Article14. Yuan J, Yeung DK, Mok GS, Bhatia KS, Wang YX, Ahuja AT, et al. Non-gaussian analysis of diffusion weighted imaging in head and neck at 3T: a pilot study in patients with nasopharyngeal carcinoma. PLoS One. 2014; 9:e87024. PMID: 24466318.
Article15. Jansen JF, Stambuk HE, Koutcher JA, Shukla-Dave A. Non-gaussian analysis of diffusion-weighted MR imaging in head and neck squamous cell carcinoma: a feasibility study. AJNR Am J Neuroradiol. 2010; 31:741–748. PMID: 20037133.
Article16. Chen Y, Ren W, Zheng D, Zhong J, Liu X, Yue Q, et al. Diffusion kurtosis imaging predicts neoadjuvant chemotherapy responses within 4 days in advanced nasopharyngeal carcinoma patients. J Magn Reson Imaging. 2015; 42:1354–1361. PMID: 25873208.
Article17. Sakamoto J, Kuribayashi A, Kotaki S, Fujikura M, Nakamura S, Kurabayashi T. Application of diffusion kurtosis imaging to odontogenic lesions: analysis of the cystic component. J Magn Reson Imaging. 2016; 44:1565–1571. PMID: 27185307.
Article18. Xu XQ, Liu J, Hu H, Su GY, Zhang YD, Shi HB, et al. Improve the image quality of orbital 3 T diffusion-weighted magnetic resonance imaging with readout-segmented echo-planar imaging. Clin Imaging. 2016; 40:793–796. PMID: 27317226.
Article19. Koyasu S, Iima M, Umeoka S, Morisawa N, Porter DA, Ito J, et al. The clinical utility of reduced-distortion readout-segmented echo-planar imaging in the head and neck region: initial experience. Eur Radiol. 2014; 24:3088–3096. PMID: 25117744.
Article20. Yuan M, Zhang YD, Zhu C, Yu TF, Shi HB, Shi ZF, et al. Comparison of intravoxel incoherent motion diffusion-weighted MR imaging with dynamic contrast-enhanced MRI for differentiating lung cancer from benign solitary pulmonary lesions. J Magn Reson Imaging. 2016; 43:669–679. PMID: 26340144.
Article21. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.
Article22. Jiang JX, Tang ZH, Zhong YF, Qiang JW. Diffusion kurtosis imaging for differentiating between the benign and malignant sinonasal lesions. J Magn Reson Imaging. 2017; 45:1446–1454. PMID: 27758016.
Article23. Roethke MC, Kuder TA, Kuru TH, Fenchel M, Hadaschik BA, Laun FB, et al. Evaluation of diffusion kurtosis imaging versus standard diffusion imaging for detection and grading of peripheral zone prostate cancer. Invest Radiol. 2015; 50:483–489. PMID: 25867657.
Article24. Jiang R, Jiang J, Zhao L, Zhang J, Zhang S, Yao Y, et al. Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget. 2015; 6:42380–42393. PMID: 26544514.
Article25. Iima M, Yano K, Kataoka M, Umehana M, Murata K, Kanao S, et al. Quantitative non-gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Invest Radiol. 2015; 50:205–211. PMID: 25260092.26. Yu J, Huang DY, Li Y, Dai X, Shi HB. Correlation of standard diffusion-weighted imaging and diffusion kurtosis imaging with distant metastases of rectal carcinoma. J Magn Reson Imaging. 2016; 44:221–229. PMID: 26715111.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thyroid-Associated Orbitopathy: Evaluating Microstructural Changes of Extraocular Muscles and Optic Nerves Using Readout-Segmented Echo-Planar Imaging-Based Diffusion Tensor Imaging
- Comparison of Monoexponential, Biexponential, Stretched-Exponential, and Kurtosis Models of Diffusion-Weighted Imaging in Differentiation of Renal Solid Masses
- Analysis of Apparent Diffusion Coefficients of the Brain in Healthy Controls: A Comparison Study between Single-Shot Echo-Planar Imaging and Read-out-Segmented Echo-Planar Imaging
- Readout-Segmented Echo-Planar Imaging in Diffusion-Weighted MR Imaging in Breast Cancer: Comparison with Single-Shot Echo-Planar Imaging in Image Quality
- Ultrafast Magnetic Resonance Imaging: Echo Planar Imaging and Spiral Scan Imaging