J Nutr Health.
2018 Feb;51(1):14-22. 10.4163/jnh.2018.51.1.14.
Effects of luteolin on chemical induced colon carcinogenesis in high fat diet-fed obese mouse
- Affiliations
-
- 1Department of Food Science and Nutrition, Daegu Catholic University, Gyeongsan 38430, Korea. kimeunj@cu.ac.kr
- KMID: 2410321
- DOI: http://doi.org/10.4163/jnh.2018.51.1.14
Abstract
- PURPOSE
Colorectal cancer, which is one of the most commonly diagnosed cancers in developing and developed countries, is highly associated with obesity. The association is largely attributed to changes to western style diets in those countries containing high-fat and high-energy. Luteolin (LUT) is a known potent inhibitor of inflammation, obesity, and cancer. In this study, we investigated the effects of LUT on chemical-induced colon carcinogenesis in high fat diet (HFD)-fed obese mice.
METHODS
Five-week-old male C57BL/6 mice received a single intraperitoneal injection of azoxymethane (AOM) at a dose of 12.5 mg/kg body weight. Mice were then divided into four groups (n = 10) that received one of the following diets for 11 weeks after the AOM injection: normal diet (ND); HFD; HFD with 0.0025% LUT (HFD LL); HFD with 0.005% LUT (HFD HL). One week after AOM injection, animals received 1~2% dextran sodium sulfate in their drinking water over three cycles consisting of five consecutive days each that were separated by 16 days.
RESULTS
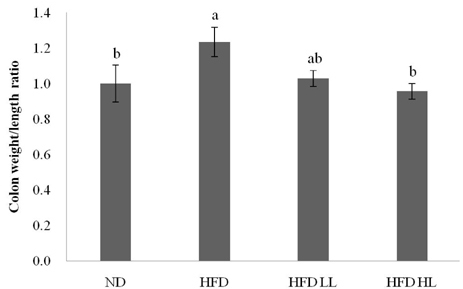
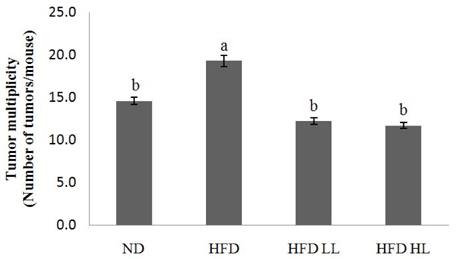
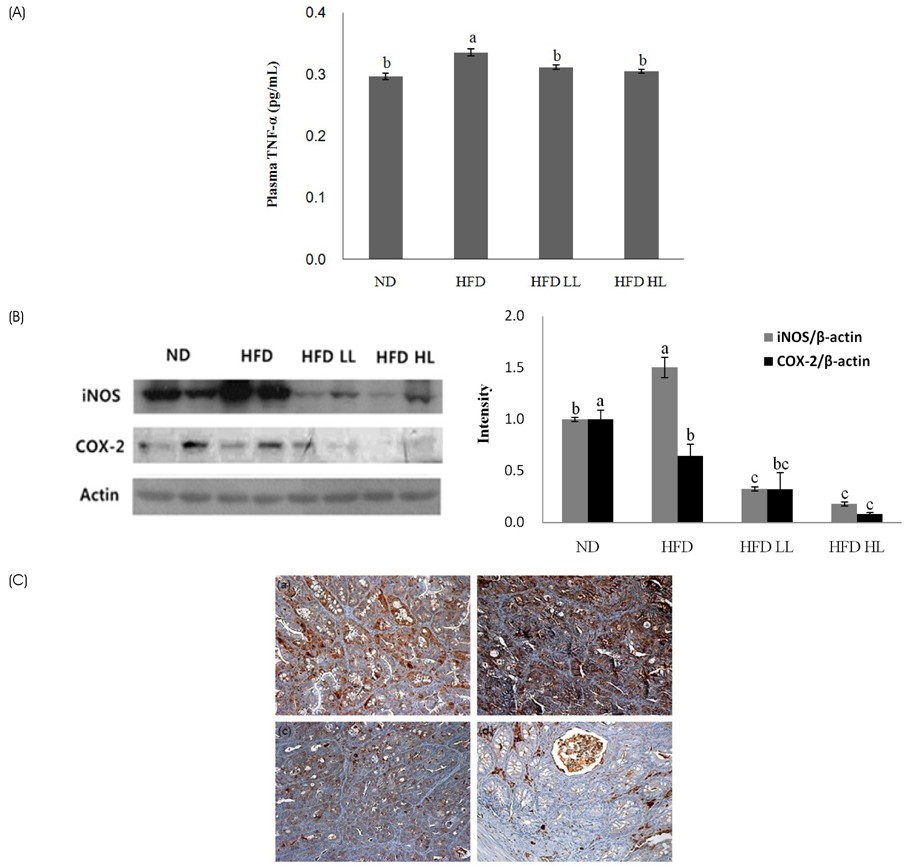
Body weight, ratio of colon weight/length, and tumor multiplicity increased significantly in the HFD group compared to the ND group. Luteolin supplementation of the HFD significantly reduced the ratio of colon weight/length and colon tumors, but not body weight. The levels of plasma TNF-α and colonic expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 protein increased in response to HFD, but were suppressed by LUT supplementation. Immunohistochemistry analysis also showed that iNOS expression was decreased by LUT.
CONCLUSION
Consumption of LUT may reduce the risk of obesity-associated colorectal cancer by suppression of colonic inflammation.
Keyword
MeSH Terms
-
Animals
Azoxymethane
Body Weight
Carcinogenesis*
Colon*
Colonic Neoplasms
Colorectal Neoplasms
Cyclooxygenase 2
Developed Countries
Dextrans
Diet
Diet, High-Fat
Drinking Water
Humans
Immunohistochemistry
Inflammation
Injections, Intraperitoneal
Luteolin*
Male
Mice
Mice, Obese*
Nitric Oxide Synthase Type II
Obesity
Plasma
Sodium
Azoxymethane
Cyclooxygenase 2
Dextrans
Drinking Water
Luteolin
Nitric Oxide Synthase Type II
Sodium
Figure
Reference
-
1. World Cancer Research Fund. American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, D.C.: WCRF/AICR;2007.2. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009; 22(4):191–197.
Article3. Boyle P, Langman JS. ABC of colorectal cancer: epidemiology. BMJ. 2000; 321(7264):805–808.
Article4. Young GP, Le Leu RK. Preventing cancer: dietary lifestyle or clinical intervention? Asia Pac J Clin Nutr. 2002; 11:Suppl 3. S618–S631.
Article5. Na SY, Myung SJ. Obesity and colorectal cancer. Korean J Gastroenterol. 2012; 59(1):16–26.
Article6. Padidar S, Farquharson AJ, Williams LM, Kearney R, Arthur JR, Drew JE. High-fat diet alters gene expression in the liver and colon: links to increased development of aberrant crypt foci. Dig Dis Sci. 2012; 57(7):1866–1874.
Article7. Sung MK, Yeon JY, Park SY, Park JH, Choi MS. Obesity-induced metabolic stresses in breast and colon cancer. Ann N Y Acad Sci. 2011; 1229:61–68.
Article8. Schlesinger S, Lieb W, Koch M, Fedirko V, Dahm CC, Pischon T, Nöthlings U, Boeing H, Aleksandrova K. Body weight gain and risk of colorectal cancer: a systematic review and meta-analysis of observational studies. Obes Rev. 2015; 16(7):607–619.
Article9. Liu Z, Brooks RS, Ciappio ED, Kim SJ, Crott JW, Bennett G, Greenberg AS, Mason JB. Diet-induced obesity elevates colonic TNF-α in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem. 2012; 23(10):1207–1213.
Article10. Jochem C, Leitzmann M. Obesity and colorectal cancer. Recent Results Cancer Res. 2016; 208:17–41.
Article11. López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem. 2009; 9(1):31–59.12. Ashokkumar P, Sudhandiran G. Protective role of luteolin on the status of lipid peroxidation and antioxidant defense against azoxymethane-induced experimental colon carcinogenesis. Biomed Pharmacother. 2008; 62(9):590–597.
Article13. Nishitani Y, Yamamoto K, Yoshida M, Azuma T, Kanazawa K, Hashimoto T, Mizuno M. Intestinal anti-inflammatory activity of luteolin: role of the aglycone in NF-κB inactivation in macrophages co-cultured with intestinal epithelial cells. Biofactors. 2013; 39(5):522–533.
Article14. Salib JY, Michael HN, Eskande EF. Anti-diabetic properties of flavonoid compounds isolated from Hyphaene thebaica epicarp on alloxan induced diabetic rats. Pharmacognosy Res. 2013; 5(1):22–29.
Article15. Pandurangan AK, Esa NM. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: a review. Asian Pac J Cancer Prev. 2014; 15(14):5501–5508.
Article16. Zhang X, Zhang QX, Wang X, Zhang L, Qu W, Bao B, Liu CA, Liu J. Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1α pathway-mediated mechanism. Int J Obes (Lond). 2016; 40(12):1841–1849.
Article17. Kwon EY, Jung UJ, Park T, Yun JW, Choi MS. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes. 2015; 64(5):1658–1669.
Article18. Zhang L, Han YJ, Zhang X, Wang X, Bao B, Qu W, Liu J. Luteolin reduces obesity-associated insulin resistance in mice by activating AMPKα1 signalling in adipose tissue macrophages. Diabetologia. 2016; 59(10):2219–2228.
Article19. Ding L, Jin D, Chen X. Luteolin enhances insulin sensitivity via activation of PPARγ transcriptional activity in adipocytes. J Nutr Biochem. 2010; 21(10):941–947.
Article20. Nepali S, Son JS, Poudel B, Lee JH, Lee YM, Kim DK. Luteolin is a bioflavonoid that attenuates adipocyte-derived inflammatory responses via suppression of nuclear factor-κ B/mitogen-activated protein kinases pathway. Pharmacogn Mag. 2015; 11(43):627–635.21. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993; 69(2):238–249.22. Cooper HS, Murthy S, Kido K, Yoshitake H, Flanigan A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000; 21(4):757–768.
Article23. Tanaka T, Kohno H, Suzuki R, Hata K, Sugie S, Niho N, Sakano K, Takahashi M, Wakabayashi K. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in ApcMin/+ mice: inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int J Cancer. 2006; 118(1):25–34.24. Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998; 115(1):182–205.
Article25. Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002; 15(1):79–94.
Article26. Renehan AG, Soerjomataram I, Tyson M, Egger M, Zwahlen M, Coebergh JW, Buchan I. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010; 126(3):692–702.
Article27. Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007; 16(12):2533–2547.
Article28. Martínez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses' Health Study Research Group. J Natl Cancer Inst. 1997; 89(13):948–955.29. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007; 86(3):556–565.
Article30. Baltgalvis KA, Berger FG, Peña MM, Davis JM, Carson JA. The interaction of a high-fat diet and regular moderate intensity exercise on intestinal polyp development in Apc Min/+ mice. Cancer Prev Res (Phila). 2009; 2(7):641–649.31. Tang FY, Pai MH, Chiang EP. Consumption of high-fat diet induces tumor progression and epithelial-mesenchymal transition of colorectal cancer in a mouse xenograft model. J Nutr Biochem. 2012; 23(10):1302–1313.
Article32. Reddy BS. Types and amount of dietary fat and colon cancer risk: Prevention by omega-3 fatty acid-rich diets. Environ Health Prev Med. 2002; 7(3):95–102.
Article33. Dai W, Liu T, Wang Q, Rao CV, Reddy BS. Down-regulation of PLK3 gene expression by types and amount of dietary fat in rat colon tumors. Int J Oncol. 2002; 20(1):121–126.
Article34. van Beelen VA, Spenkelink B, Mooibroek H, Sijtsma L, Bosch D, Rietjens IM, Alink GM. An n-3 PUFA-rich microalgal oil diet protects to a similar extent as a fish oil-rich diet against AOM-induced colonic aberrant crypt foci in F344 rats. Food Chem Toxicol. 2009; 47(2):316–320.
Article35. Kang KA, Piao MJ, Ryu YS, Hyun YJ, Park JE, Shilnikova K, Zhen AX, Kang HK, Koh YS, Jeong YJ, Hyun JW. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int J Oncol. 2017; 51(4):1169–1178.
Article36. Pandurangan AK, Dharmalingam P, Sadagopan SK, Ramar M, Munusamy A, Ganapasam S. Luteolin induces growth arrest in colon cancer cells through involvement of Wnt/β-catenin/GSK-3β signaling. J Environ Pathol Toxicol Oncol. 2013; 32(2):131–139.
Article37. Lim DY, Jeong Y, Tyner AL, Park JH. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 2007; 292(1):G66–G75.
Article38. Ramos AA, Pereira-Wilson C, Collins AR. Protective effects of ursolic acid and luteolin against oxidative DNA damage include enhancement of DNA repair in Caco-2 cells. Mutat Res. 2010; 692(1-2):6–11.
Article39. Pandurangan AK, Ananda Sadagopan SK, Dharmalingam P, Ganapasam S. Luteolin, a bioflavonoid, attenuates azoxymethane-induced effects on mitochondrial enzymes in BALB/c mice. Asian Pac J Cancer Prev. 2014; 14(11):6669–6672.
Article40. Pandurangan AK, Dharmalingam P, Sadagopan SK, Ganapasam S. Luteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesis. Hum Exp Toxicol. 2014; 33(11):1176–1185.
Article41. Rankin JW, Turpyn AD. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J Am Coll Nutr. 2007; 26(2):163–169.
Article42. Kim IW, Myung SJ, Do MY, Ryu YM, Kim MJ, Do EJ, Park S, Yoon SM, Ye BD, Byeon JS, Yang SK, Kim JH. Western-style diets induce macrophage infiltration and contribute to colitis-associated carcinogenesis. J Gastroenterol Hepatol. 2010; 25(11):1785–1794.
Article43. Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007; 28(9):1450–1459.
Article44. Park YH, Kim N, Shim YK, Choi YJ, Nam RH, Choi YJ, Ham MH, Suh JH, Lee SM, Lee CM, Yoon H, Lee HS, Lee DH. Adequate dextran sodium sulfate-induced colitis model in mice and effective outcome measurement method. J Cancer Prev. 2015; 20(4):260–267.
Article45. Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014; 18(4):279–288.
Article46. Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000; 21(7):1319–1327.47. Ahn B, Ohshima H. Suppression of intestinal polyposis in Apc(Min/+) mice by inhibiting nitric oxide production. Cancer Res. 2001; 61(23):8357–8360.48. Yagihashi N, Kasajima H, Sugai S, Matsumoto K, Ebina Y, Morita T, Murakami T, Yagihashi S. Increased in situ expression of nitric oxide synthase in human colorectal cancer. Virchows Arch. 2000; 436(2):109–114.
Article49. Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002; 53:35–57.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiproliferative properties of luteolin against chemically induced colon cancer in mice fed on a high-fat diet and colorectal cancer cells grown in adipocyte-derived medium
- The effects of Angelica keiskei Koidz on the expression of antioxidant enzymes related to lipid profiles in rats fed a high fat diet
- Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice
- Expression of eotaxin in 3T3-L1 adipocytes and the effects of weight loss in high-fat diet induced obese mice
- Effects of poly-gamma-glutamic acid on serum and brain concentrations of glutamate and GABA in diet-induced obese rats