Immune Netw.
2018 Apr;18(2):e2. 10.4110/in.2018.18.e2.
The Detailed Kinetics of Cytomegalovirus-specific T cell Responses after Hematopoietic Stem Cell Transplantation: 1 Year Follow-up Data
- Affiliations
-
- 1Department of Infectious Diseases, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea. kimsunghanmd@hotmail.com
- 2Division of Infectious Diseases, Department of Internal Medicine, Ulsan University Hospital, Ulsan 44033, Korea.
- 3Department of Hematology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea.
- 4Department of Laboratory Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea.
- KMID: 2410155
- DOI: http://doi.org/10.4110/in.2018.18.e2
Abstract
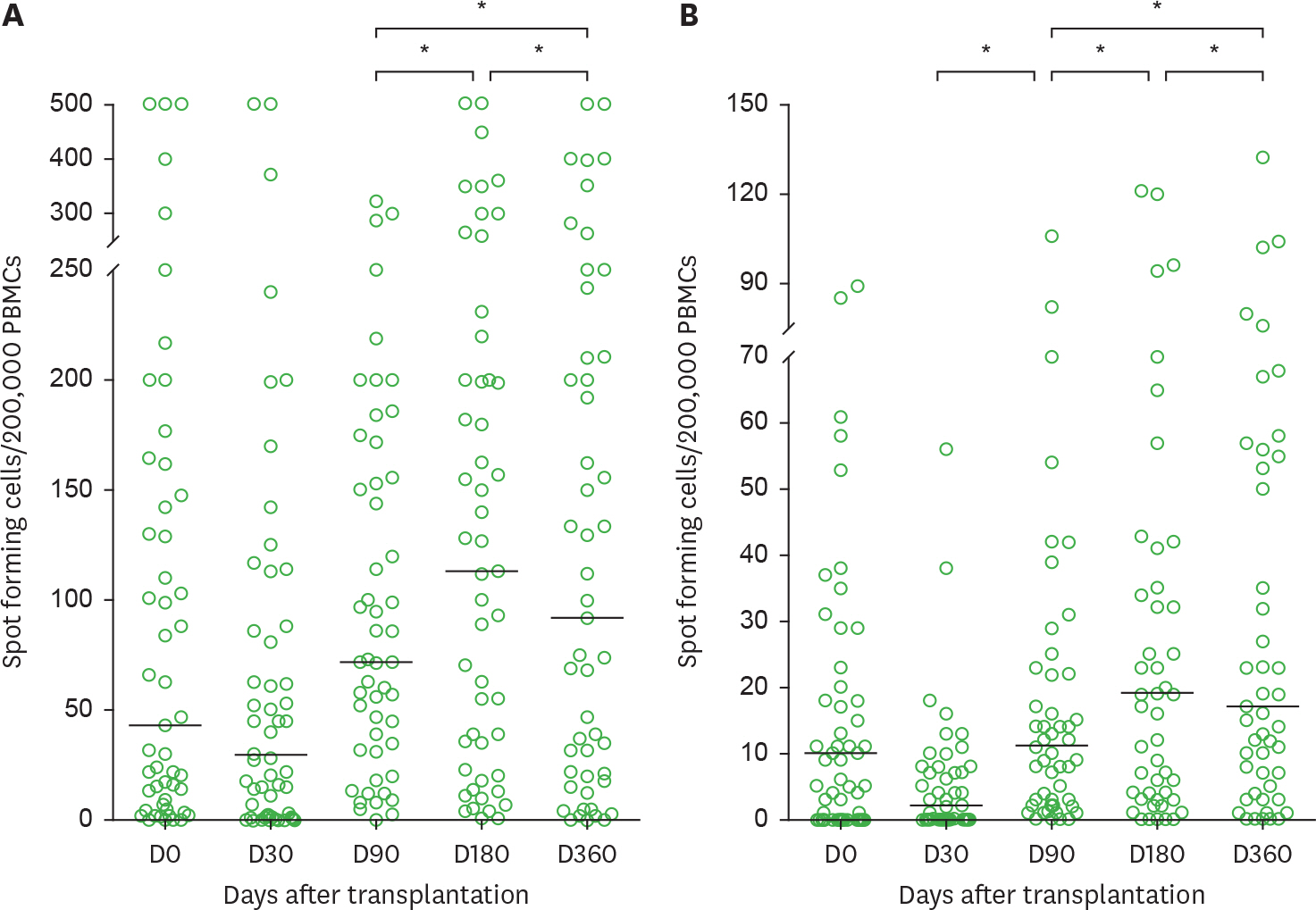
- The detailed kinetics of the cytomegalovirus (CMV)-specific T cell response in hematopoietic stem cell transplant (HCT) recipients have not yet been fully assessed. We evaluated these kinetics of CMV-specific T cell response and factors associated with high CMV-specific T cell responses 1 year after HCT. In HCT recipients, CMV pp65 and IE1-specific ELISPOT assay were performed before HCT (D0), and at 30 (D30), 90 (D90), 180 (D180), and 360 (D360) days after HCT. Of the 51 HCT recipients with donor-positive (D+)/recipient-positive (R+) serology, 26 (51%) developed CMV infections after HCT. The patterns of post-transplantation reconstitution for CMV-specific T cell response were classified into 4 types: 1) an initial decrease at D30 followed by gradual T cell reconstitution without CMV infection (35%), 2) an initial decrease at D30 followed by gradual T cell reconstitution preceded by CMV infection (35%), 3) failure of gradual or constant T cell reconstitution (26%), and 4) no significant T cell reconstitution (4%). There was no significant difference between ELISPOT counts of D360 and those of D0. High CMV-specific T cell responses at D360 were not associated with high CMV-specific T cell response at D0, CMV infection, ganciclovir therapy, graft versus host disease (GVHD), and immunosuppressant use. In conclusion, there are 4 distinct patterns of reconstitution of the CMV-specific T cell response after HCT. In addition, reconstituted donor-origin CMV-specific T cell responses appeared to be constant until day 360 after HCT, regardless of the level of the pre-transplant CMV-specific T cell response, CMV infection, and immunosuppressant use.
MeSH Terms
Figure
Reference
-
References
1. Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991; 78:1373–1380.
Article2. Barron MA, Gao D, Springer KL, Patterson JA, Brunvand MW, McSweeney PA, Zeng C, Barón AE, Weinberg A. Relationship of reconstituted adaptive and innate cytomegalovirus (CMV)-specific immune responses with CMV viremia in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009; 49:1777–1783.
Article3. Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996; 88:4063–4071.
Article4. Einsele H, Hebart H, Kauffmann-Schneider C, Sinzger C, Jahn G, Bader P, Klingebiel T, Dietz K, Löffler J, Bokemeyer C, et al. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant. 2000; 25:757–763.
Article5. Green ML, Leisenring W, Stachel D, Pergam SA, Sandmaier BM, Wald A, Corey L, Boeckh M. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012; 18:1687–1699.
Article6. Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994; 83:1971–1979.
Article7. Ciáurriz M, Zabalza A, Beloki L, Mansilla C, Pérez-Valderrama E, Lachén M, Bandrés E, Olavarría E, Ramírez N. The immune response to cytomegalovirus in allogeneic hematopoietic stem cell transplant recipients. Cell Mol Life Sci. 2015; 72:4049–4062.
Article8. Hebart H, Daginik S, Stevanovic S, Grigoleit U, Dobler A, Baur M, Rauser G, Sinzger C, Jahn G, Loeffler J, et al. Sensitive detection of human cytomegalovirus peptide-specific cytotoxic T-lymphocyte responses by interferon-gamma-enzyme-linked immunospot assay and flow cytometry in healthy individuals and in patients after allogeneic stem cell transplantation. Blood. 2002; 99:3830–3837.9. Ohnishi M, Sakurai T, Heike Y, Yamazaki R, Kanda Y, Takaue Y, Mizoguchi H, Kawakami Y. Evaluation of cytomegalovirus-specific T-cell reconstitution in patients after various allogeneic haematopoietic stem cell transplantation using interferon-gamma-enzyme-linked immunospot and human leucocyte antigen tetramer assays with an immunodominant T-cell epitope. Br J Haematol. 2005; 131:472–479.
Article10. Avetisyan G, Aschan J, Hägglund H, Ringdén O, Ljungman P. Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT. Bone Marrow Transplant. 2007; 40:865–869.
Article11. Abate D, Cesaro S, Cofano S, Fiscon M, Saldan A, Varotto S, Mengoli C, Pillon M, Calore E, Biasolo MA, et al. Diagnostic utility of human cytomegalovirus-specific T-cell response monitoring in predicting viremia in pediatric allogeneic stem-cell transplant patients. Transplantation. 2012; 93:536–542.
Article12. Ljungman P, Lewensohn-Fuchs I, Hammarström V, Aschan J, Brandt L, Bolme P, Lönnqvist B, Johansson N, Ringdén O, Gahrton G. Long-term immunity to measles, mumps, and rubella after allogeneic bone marrow transplantation. Blood. 1994; 84:657–663.
Article13. Spoulou V, Giannaki M, Vounatsou M, Bakoula C, Grafakos S. Long-term immunity to measles, mumps and rubella after MMR vaccination among children with bone marrow transplants. Bone Marrow Transplant. 2004; 33:1187–1190.
Article14. Lacey SF, Diamond DJ, Zaia JA. Assessment of cellular immunity to human cytomegalovirus in recipients of allogeneic stem cell transplants. Biol Blood Marrow Transplant. 2004; 10:433–447.
Article15. Lehmann PV, Zhang W. Unique strengths of ELISPOT for T cell diagnostics. Methods Mol Biol. 2012; 792:3–23.
Article16. Luo XH, Huang XJ, Li D, Liu KY, Xu LP, Liu DH. Immune reconstitution to cytomegalovirus following partially matched-related donor transplantation: impact of in vivo T-cell depletion and granulocyte colony-stimulating factor-primed peripheral blood/bone marrow mixed grafts. Transpl Infect Dis. 2013; 15:22–33.17. Snyder LD, Chan C, Kwon D, Yi JS, Martissa JA, Copeland CA, Osborne RJ, Sparks SD, Palmer SM, Weinhold KJ. Polyfunctional T-Cell signatures to predict protection from cytomegalovirus after lung transplantation. Am J Respir Crit Care Med. 2016; 193:78–85.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary Cytomegalovirus Peritonitis Following Unrelated Hematopoietic Stem Cell Transplantation
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Hematopoietic Stem Cell Transplantation
- Hematopoietic stem cell transplantation: overview for general pediatrician
- Hematopoietic Stem Cell Transplantation in Inborn Error of Metabolism