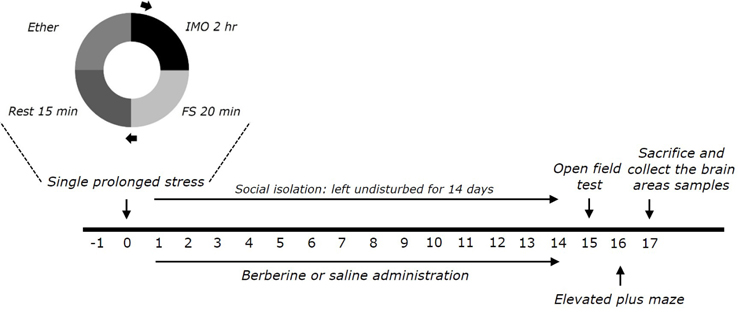
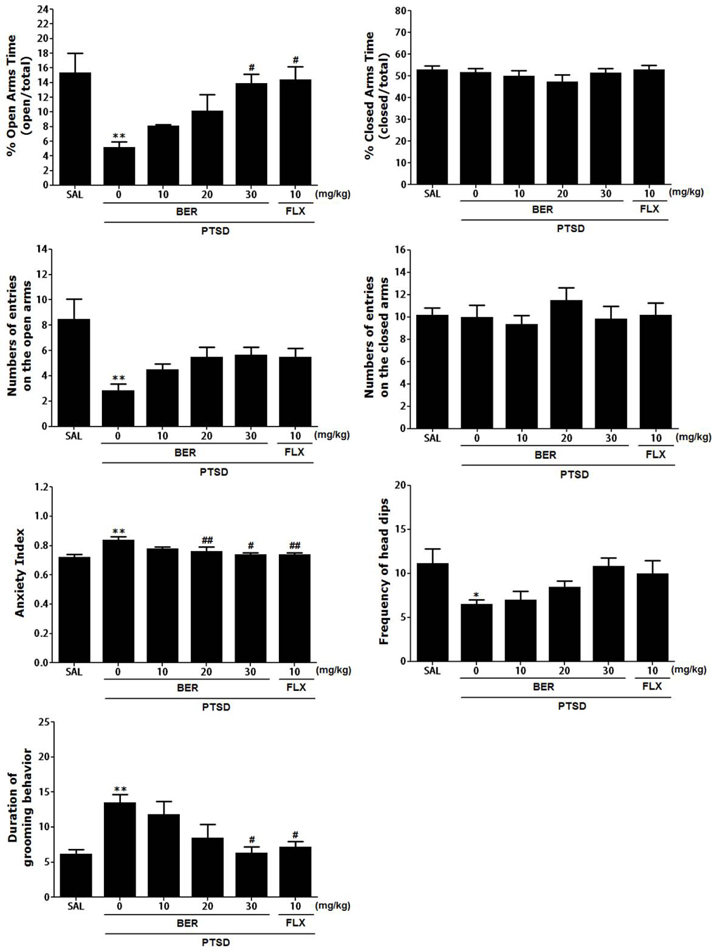
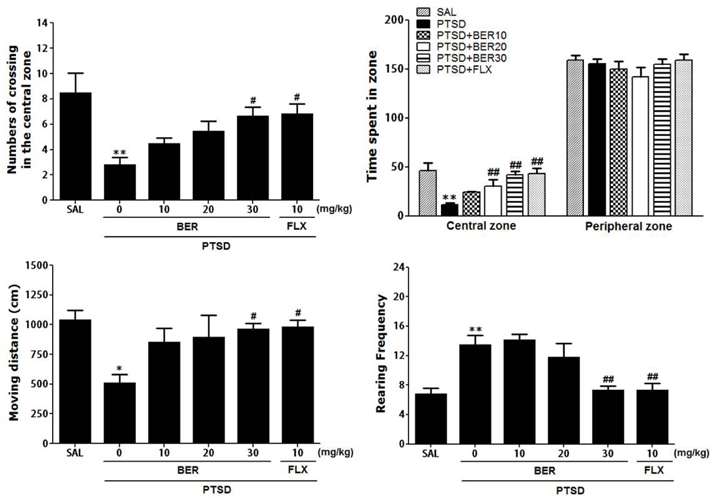
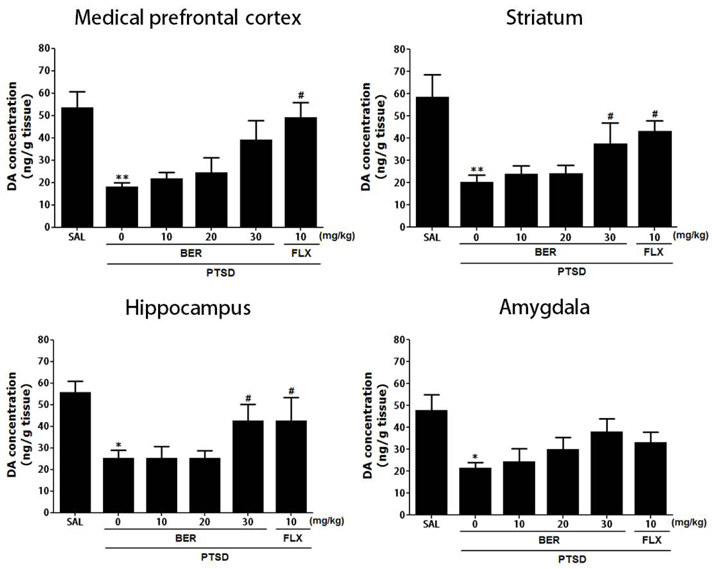
Korean J Physiol Pharmacol.
2018 Mar;22(2):183-192. 10.4196/kjpp.2018.22.2.183.
Berberine alleviates symptoms of anxiety by enhancing dopamine expression in rats with post-traumatic stress disorder
- Affiliations
-
- 1Acupuncture and Meridian Science Research Center, College of Korean Medicine, Kyung Hee University, Seoul 02447, Korea. bombi@khu.ac.kr
- 2Center for Converging Humanities, College of Medicine, Kyung Hee University, Seoul 02447, Korea.
- 3Department of Physiology, College of Medicine, Kyung Hee University, Seoul 02447, Korea. dhhahm@khu.ac.kr
- KMID: 2410108
- DOI: http://doi.org/10.4196/kjpp.2018.22.2.183
Abstract
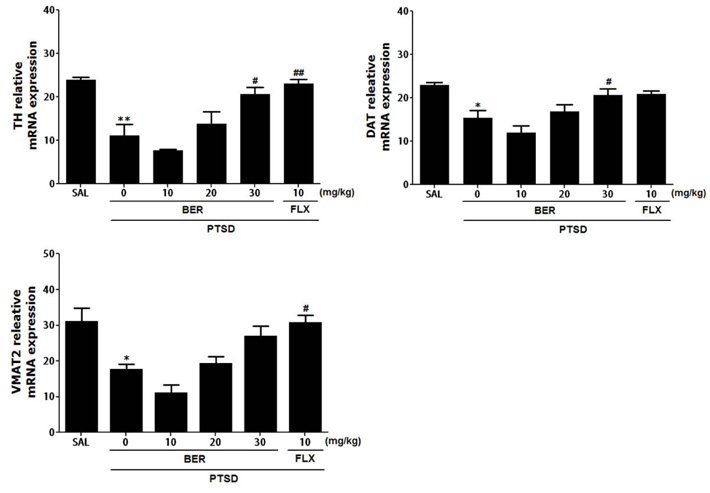
- Post-traumatic stress disorder (PTSD) is a trauma-induced psychiatric disorder characterized by impaired fear extermination, hyperarousal, anxiety, depression, and amnesic symptoms that may involve the release of monoamines in the fear circuit. The present study measured several anxiety-related behavioral responses to examine the effects of berberine (BER) on symptoms of anxiety in rats after single prolonged stress (SPS) exposure, and to determine if BER reversed the dopamine (DA) dysfunction. Rats received BER (10, 20, or 30 mg/kg, intraperitoneally, once daily) for 14 days after SPS exposure. BER administration significantly increased the time spent in the open arms and reduced grooming behavior during the elevated plus maze test, and increased the time spent in the central zone and the number of central zone crossings in the open field test. BER restored neurochemical abnormalities and the SPS-induced decrease in DA tissue levels in the hippocampus and striatum. The increased DA concentration during BER treatment may partly be attributed to mRNA expression of tyrosine hydroxylase and the DA transporter in the hippocampus, while BER exerted no significant effects on vesicular monoamine transporter mRNA expression in the hippocampus of rats with PTSD. These results suggest that BER had anxiolytic-like effects on behavioral and biochemical measures associated with anxiety. These findings support a role for reduced anxiety altered DAergic transmission and reduced anxiety in rats with PTSD. Thus, BER may be a useful agent to treat or alleviate psychiatric disorders like those observed in patients with PTSD.
MeSH Terms
Figure
Reference
-
1. Enman NM, Arthur K, Ward SJ, Perrine SA, Unterwald EM. Anhedonia, reduced cocaine reward, and dopamine dysfunction in a rat model of posttraumatic stress disorder. Biol Psychiatry. 2015; 78:871–879.
Article2. Wilson CB, Ebenezer PJ, McLaughlin LD, Francis J. Predator exposure/ psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PLoS One. 2014; 9:e89104.3. Ji LL, Tong L, Xu BK, Fu CH, Shu W, Peng JB, Wang ZY. Intra-hippocampal administration of ZIP alleviates depressive and anxiety-like responses in an animal model of posttraumatic stress disorder. Behav Brain Funct. 2014; 10–28.
Article4. Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005; 29:1207–1223.
Article5. Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010; 35:169–191.
Article6. Hadad NA, Wu L, Hiller H, Krause EG, Schwendt M, Knackstedt LA. Conditioned stress prevents cue-primed cocaine reinstatement only in stress-responsive rats. Stress. 2016; 19:406–418.
Article7. Lin CC, Tung CS, Lin PH, Huang CL, Liu YP. Traumatic stress causes distinctive effects on fear circuit catecholamines and the fear extinction profile in a rodent model of posttraumatic stress disorder. Eur Neuropsychopharmacol. 2016; 26:1484–1495.
Article8. Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012; 13:769–787.
Article9. Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009; 26:1110–1117.
Article10. Eskandarian S, Vafaei AA, Vaezi GH, Taherian F, Kashefi A, Rashidy-Pour A. Effects of systemic administration of oxytocin on contextual fear extinction in a rat model of post-traumatic stress disorder. Basic Clin Neurosci. 2013; 4:315–322.11. Kohda K, Harada K, Kato K, Hoshino A, Motohashi J, Yamaji T, Morinobu S, Matsuoka N, Kato N. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience. 2007; 148:22–33.
Article12. Pitman RK. Overview of biological themes in PTSD. Ann N Y Acad Sci. 1997; 821:1–9.
Article13. Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005; 30:162–178.
Article14. Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. L-tetrahydropalmatine ameliorates development of anxiety and depression-related symptoms induced by single prolonged stress in rats. Biomol Ther (Seoul). 2014; 22:213–222.15. Gemmel M, Rayen I, van Donkelaar E, Loftus T, Steinbusch HW, Kokras N, Dalla C, Pawluski JL. Gestational stress and fluoxetine treatment differentially affect plasticity, methylation and serotonin levels in the PFC and hippocampus of rat dams. Neuroscience. 2016; 327:32–43.
Article16. Garabadu D, Ahmad A, Krishnamurthy S. Risperidone attenuates modified stress-re-stress paradigm-induced mitochondrial dysfunction and apoptosis in rats exhibiting post-traumatic stress disorder-like symptoms. J Mol Neurosci. 2015; 56:299–312.
Article17. Liberzon I, López JF, Flagel SB, Vázquez DM, Young EA. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol. 1999; 11:11–17.
Article18. Nie H, Peng Z, Lao N, Wang H, Chen Y, Fang Z, Hou W, Gao F, Li X, Xiong L, Tan Q. Rosmarinic acid ameliorates PTSD-like symptoms in a rat model and promotes cell proliferation in the hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2014; 51:16–22.
Article19. Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. Effect of berberine on depression- and anxiety-like behaviors and activation of the noradrenergic system induced by development of morphine dependence in rats. Korean J Physiol Pharmacol. 2012; 16:379–386.
Article20. Hwang YH, Cho WK, Jang D, Ha JH, Jung K, Yun HI, Ma JY. Effects of berberine and hwangryunhaedok-tang on oral bioavailability and pharmacokinetics of ciprofloxacin in rats. Evid Based Complement Alternat Med. 2012; 2012:673132.
Article21. Moghaddam HK, Baluchnejadmojarad T, Roghani M, Khaksari M, Norouzi P, Ahooie M, Mahboobi F. Berberine ameliorate oxidative stress and astrogliosis in the hippocampus of STZ-induced diabetic rats. Mol Neurobiol. 2014; 49:820–826.
Article22. Kwon IH, Choi HS, Shin KS, Lee BK, Lee CK, Hwang BY, Lim SC, Lee MK. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson's disease. Neurosci Lett. 2010; 486:29–33.
Article23. Qin-Wei Z, Yong-Guang LI. Berberine attenuates myocardial ischemia reperfusion injury by suppressing the activation of PI3K/AKT signaling. Exp Ther Med. 2016; 11:978–984.
Article24. Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer's disease. BMC Neurosci. 2006; 78–87.
Article25. Mantsch JR, Li SJ, Risinger R, Awad S, Katz E, Baker DA, Yang Z. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology (Berl). 2007; 192:581–591.
Article26. Yun J. L-tetrahydropalmatine inhibits methamphetamine-induced locomotor activity via regulation of 5-HT neuronal activity and dopamine D3 receptor expression. Phytomedicine. 2014; 21:1287–1291.
Article27. Peng WH, Wu CR, Chen CS, Chen CF, Leu ZC, Hsieh MT. Anxiolytic effect of berberine on exploratory activity of the mouse in two experimental anxiety models: interaction with drugs acting at 5-HT receptors. Life Sci. 2004; 75:2451–2462.
Article28. Patki G, Li L, Allam F, Solanki N, Dao AT, Alkadhi K, Salim S. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav. 2014; 130:47–53.
Article29. Lee B, Sur B, Cho SG, Yeom M, Shim I, Lee H, Hahm DH. Ginsenoside Rb1 rescues anxiety-like responses in a rat model of posttraumatic stress disorder. J Nat Med. 2016; 70:133–144.
Article30. Yeom M, Sur BJ, Park J, Cho SG, Lee B, Kim ST, Kim KS, Lee H, Hahm DH. Oral administration of Lactobacillus casei variety rhamnosus partially alleviates TMA-induced atopic dermatitis in mice through improving intestinal microbiota. J Appl Microbiol. 2015; 119:560–570.
Article31. Eagle AL, Fitzpatrick CJ, Perrine SA. Single prolonged stress impairs social and object novelty recognition in rats. Behav Brain Res. 2013; 256:591–597.
Article32. Peng Y, Feng SF, Wang Q, Wang HN, Hou WG, Xiong L, Luo ZJ, Tan QR. Hyperbaric oxygen preconditioning ameliorates anxiety-like behavior and cognitive impairments via upregulation of thioredoxin reductases in stressed rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010; 34:1018–1025.
Article33. Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I. Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem. 2012; 19:43–49.
Article34. Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008; 33:2108–2116.
Article35. Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl). 2004; 172:225–229.
Article36. Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathé AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012; 37:350–363.
Article37. Serova LI, Laukova M, Alaluf LG, Sabban EL. Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behav Brain Res. 2013; 250:139–147.
Article38. Lin CC, Tung CS, Liu YP. Escitalopram reversed the traumatic stress-induced depressed and anxiety-like symptoms but not the deficits of fear memory. Psychopharmacology (Berl). 2016; 233:1135–1146.
Article39. Zhang XG, Zhang H, Liang XL, Liu Q, Wang HY, Cao B, Cao J, Liu S, Long YJ, Xie WY, Peng DZ. Epigenetic mechanism of maternal post-traumatic stress disorder in delayed rat offspring development: dysregulation of methylation and gene expression. Genet Mol Res. 2016; 15:15039009.
Article40. Wang HT, Han F, Shi YX. Activity of the 5-HT1A receptor is involved in the alteration of glucocorticoid receptor in hippocampus and corticotropin-releasing factor in hypothalamus in SPS rats. Int J Mol Med. 2009; 24:227–231.
Article41. Aykaç A, AydXMLLink_XYZn B, Cabadak H, Gören MZ. The change in muscarinic receptor subtypes in different brain regions of rats treated with fluoxetine or propranolol in a model of post-traumatic stress disorder. Behav Brain Res. 2012; 232:124–129.
Article42. Jichao S, Xinmin H, Xianguo R, Dongqi Y, Rongyi Z, Shuang L, Yue Y, Yuchen S, Jingnan Y. Saikosaponin a alleviates symptoms of attention deficit hyperactivity disorder through downregulation of DAT and enhancing BDNF expression in spontaneous hypertensive rats. Evid Based Complement Alternat Med. 2017; 2017:2695903.
Article43. Nikolaus S, Antke C, Beu M, Müller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders−results from in vivo imaging studies. Rev Neurosci. 2010; 21:119–139.
Article44. Kim M, Cho KH, Shin MS, Lee JM, Cho HS, Kim CJ, Shin DH, Yang HJ. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson's disease. Int J Mol Med. 2014; 33:870–878.
Article45. Negahdar F, Mehdizadeh M, Joghataei MT, Roghani M, Mehraeen F, Poorghayoomi E. Berberine chloride pretreatment exhibits neuroprotective effect against 6-hydroxydopamine-induced neuronal insult in rat. Iran J Pharm Res. 2015; 14:1145–1152.46. Bae J, Lee D, Kim YK, Gil M, Lee JY, Lee KJ. Berberine protects 6-hydroxydopamine-induced human dopaminergic neuronal cell death through the induction of heme oxygenase-1. Mol Cells. 2013; 35:151–157.
Article47. Shin JS, Kim EI, Kai M, Lee MK. Inhibition of dopamine biosynthesis by protoberberine alkaloids in PC12 cells. Neurochem Res. 2000; 25:363–368.48. Sun H, Zhu L, Yang H, Qian W, Guo L, Zhou S, Gao B, Li Z, Zhou Y, Jiang H, Chen K, Zhen X, Liu H. Asymmetric total synthesis and identification of tetrahydroprotoberberine derivatives as new antipsychotic agents possessing a dopamine D1, D2 and serotonin 5-HT1A multi-action profile. Bioorg Med Chem. 2013; 21:856–868.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Post-Traumatic Stress Disorder, Depression and Anxiety among North Korean Refugees: A Meta-Analysis
- Plasma Homovanillic Acid Level in Posttraumatic Stress Disorder
- Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder
- Prevalence and Risk Factors of Anxiety, Depression, and Post-Traumatic Stress Disorder in Critical Care Survivors
- L-Tetrahydropalmatine Ameliorates Development of Anxiety and Depression-Related Symptoms Induced by Single Prolonged Stress in Rats